Intracardiac vs Transesophageal Echocardiography for Left Atrial Appendage Occlusion With Watchman FLX in the U.S
- PMID: 37831030
- PMCID: PMC12337774
- DOI: 10.1016/j.jacep.2023.08.004
Intracardiac vs Transesophageal Echocardiography for Left Atrial Appendage Occlusion With Watchman FLX in the U.S
Abstract
Background: Intraprocedural imaging is critical for device delivery in transcatheter left atrial appendage occlusion (LAAO). Although pivotal trials of LAAO devices were conducted using transesophageal echocardiography (TEE), intracardiac echocardiography (ICE) is an emerging imaging modality.
Objectives: This study compared outcomes after ICE- and TEE-guided Watchman FLX implantation in the SURPASS (SURveillance Post Approval AnalySiS Plan) nationwide LAAO registry.
Methods: Baseline characteristics were compared using chi-square and t-tests. Outcomes were reported in unadjusted and adjusted comparisons via propensity weighting.
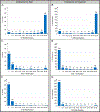
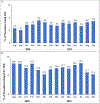
Results: Between August 2020 and September 2021, LAAO was attempted in 39,759 patients at 698 sites, including 2,272 cases (5.7%) with ICE and 31,835 (80.0%) with TEE. ICE and TEE patients had similar baseline characteristics and mean procedural times (ICE 82 minutes vs TEE 78 minutes). ICE patients were less likely to receive general anesthesia (54% vs 98%, P < 0.01). Successful device implantation (98.3% vs 97.6%) and complete seal rates at 45 days were similar (n = 25,280; 83% vs 82%). Most adverse event rates were similar; unadjusted mortality rates at 45 days were 1.1% for ICE vs 0.8% for TEE (P = 0.14), and 1.0% vs 0.7% (P = 0.27) in adjusted analyses. Even after adjustment, pericardial effusion rates requiring intervention were significantly higher with ICE at 45 days (1.0% vs 0.5%; P = 0.02). This rate decreased as operators performed more ICE-guided procedures, although 82% of operators had performed <10 ICE-guided procedures overall.
Conclusions: In the largest comparison to date, ICE use was infrequent. ICE and TEE both achieved high rates of complete LAAO. ICE was associated with significantly higher rates of pericardial effusion requiring intervention.
Keywords: atrial fibrillation; intracardiac echocardiography; left atrial appendage occlusion; pericardial effusion; peridevice leak; transesophageal echocardiography.
Copyright © 2023 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The SURPASS analysis was funded by the Boston Scientific Corporation. The views expressed here represent those of the authors, and do not necessarily represent the official views of the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR) or its associated professional societies identified at CVQuality.ACC.org/NCDR. Dr Piccini is supported by R01AG074185 from the National Institutes of Aging. Dr Hsu has received honoraria from Medtronic, Abbott, Boston Scientific, Biotronik, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Sanofi, Zoll Medical, iRhythm, Acutus Medical, Galvanize Therapeutics, and Biosense-Webster; research grants from Biotronik and Biosense-Webster; and has equity interest in Vektor Medical. Dr Freeman has received research funding from the NIH/NHLBI and the American College of Cardiology National Cardiovascular Data Registry; consulting/advisory board fees from Medtronic, Boston Scientific, Biosense Webster, and PaceMate; and equity in PaceMate. Dr Price has received consulting honoraria, speaker fees, and proctoring fees from Abbott Vascular and Boston Scientific; consulting honoraria from W. L. Gore & Associates, Baylis Medical, Biotronik, and Philips; consulting honoraria and speaker fees from Medtronic; consulting honoraria from Biosense Webster and Shockwave; and has equity in Indian Wells, Inc. Drs Allocco and Roy are full-time employees and stockholders of Boston Scientific. Dr Yeh has received research funding and consulting fees from Abbott Vascular, Boston Scientific, and Medtronic; and research funding from Bard, Cook, and Philips. Dr Piccini has received grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, iRhythm, and Philips; and serves as a consultant to Abbott, Abbvie, ARCA biopharma, Bayer, Boston Scientific, Bristol Myers Squibb (Myokardia), Element Science, Itamar Medical, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, ReCor, Sanofi, Philips, and Up-to-Date. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Comment in
-
Intracardiac Echo for Left Atrial Appendage Closure: Will Practice Make Perfect?JACC Clin Electrophysiol. 2023 Dec;9(12):2600-2602. doi: 10.1016/j.jacep.2023.10.027. JACC Clin Electrophysiol. 2023. PMID: 38151304 No abstract available.
References
-
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. Jul 8 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

