Maternal-fetal cross-talk via the placenta: influence on offspring development and metabolism
- PMID: 37831056
- PMCID: PMC10617615
- DOI: 10.1242/dev.202088
Maternal-fetal cross-talk via the placenta: influence on offspring development and metabolism
Abstract
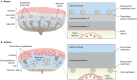
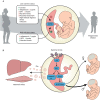
Compelling epidemiological and animal experimental data demonstrate that cardiometabolic and neuropsychiatric diseases originate in a suboptimal intrauterine environment. Here, we review evidence suggesting that altered placental function may, at least in part, mediate the link between the maternal environment and changes in fetal growth and development. Emerging evidence indicates that the placenta controls the development and function of several fetal tissues through nutrient sensing, modulation of trophoblast nutrient transporters and by altering the number and cargo of released extracellular vesicles. In this Review, we discuss the development and functions of the maternal-placental-fetal interface (in humans and mice) and how cross-talk between these compartments may be a mechanism for in utero programming, focusing on mechanistic target of rapamycin (mTOR), adiponectin and O-GlcNac transferase (OGT) signaling. We also discuss how maternal diet and stress influences fetal development and metabolism and how fetal growth restriction can result in susceptibility to developing chronic disease later in life. Finally, we speculate how interventions targeting placental function may offer unprecedented opportunities to prevent cardiometabolic disease in future generations.
Keywords: Epigenetics; Extracellular vesicles; Fetal development; Maternal-fetal exchange; Prenatal; Programming.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Akhaphong, B., Baumann, D. C., Beetch, M., Lockridge, A. D., Jo, S., Wong, A., Zemanovic, T., Mohan, R., Fondevilla, D. L., Sia, M.et al. (2021). Placental mTOR complex 1 regulates fetal programming of obesity and insulin resistance in mice. JCI Insight 6, e149271. 10.1172/jci.insight.149271 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

