Phase II study of apatinib plus exemestane in estrogen receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer
- PMID: 37831547
- PMCID: PMC10578185
- DOI: 10.1080/15384047.2023.2265055
Phase II study of apatinib plus exemestane in estrogen receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer
Abstract
Purpose: Apatinib is a tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR)-2. This study was conducted to assess the efficacy and safety of apatinib combined with exemestane in patients with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (MBC).
Methods: This single-center, single-arm phase II study enrolled patients with ER+/HER2- MBC progressed on previous letrozole or anastrozole. Stratified analysis was performed according to the number of chemotherapy regimens for metastatic disease. The primary endpoint was progression free survival (PFS). Secondary endpoints included objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR), overall survival (OS) and toxicity. Patients received apatinib at a starting dose of 500 mg/d and exemestane 25 mg/d on days 1-28 of each 4-week cycle.
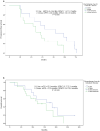
Results: Thirty patients were enrolled with median four prior anticancer therapies. Eighty percent of patients received chemotherapy for metastatic disease. The median PFS (mPFS) and OS were 5.6 (95%CI: 4.3-6.9) months and 15.7 (95% CI: 9.7-21.7) months, respectively. The ORR, DCR, and CBR were 21.4%, 71.4%, and 46.4%, respectively. Patients with 0-1 line chemotherapy for MBC showed a slightly longer mPFS compared to those with ≥2 lines chemotherapy (mPFS: 6.4 months vs 4.8 months, P = .090). Most of the AEs were grade 1/2. One patient (3.3%) who suffered bone marrow metastases experienced grade 4 thrombocytopenia, and 14 experienced grade 3 AEs. Fifty percent of patients were given reduced dose for apatinib.
Conclusions: Apatinib plus exemestane exhibited objective efficacy in patients with ER+/HER2- MBC who have failed multiple lines of treatment. The AEs of apatinib required close monitoring and most of patients were well tolerated.
Keywords: Apatinib; endocrine resistance; exemestane; metastatic breast cancer (MBC).
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
-
- Qu Z, Van Ginkel S, Roy AM, Westbrook L, Nasrin M, Maxuitenko Y, Frost AR, Carey D, Wang W, Li R, et al. Vascular endothelial growth factor reduces tamoxifen efficacy and promotes metastatic colonization and desmoplasia in breast tumors. Cancer Res. 2008;68(15):6232–6240. doi: 10.1158/0008-5472.CAN-07-5654. - DOI - PMC - PubMed
-
- Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60(11):2898–2905. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
