The Drosophila blood-brain barrier regulates sleep via Moody G protein-coupled receptor signaling
- PMID: 37831742
- PMCID: PMC10589661
- DOI: 10.1073/pnas.2309331120
The Drosophila blood-brain barrier regulates sleep via Moody G protein-coupled receptor signaling
Abstract
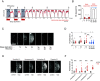
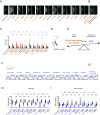
Sleep is vital for most animals, yet its mechanism and function remain unclear. We found that permeability of the BBB (blood-brain barrier)-the organ required for the maintenance of homeostatic levels of nutrients, ions, and other molecules in the brain-is modulated by sleep deprivation (SD) and can cell-autonomously effect sleep changes. We observed increased BBB permeability in known sleep mutants as well as in acutely sleep-deprived animals. In addition to molecular tracers, SD-induced BBB changes also increased the penetration of drugs used in the treatment of brain pathologies. After chronic/genetic or acute SD, rebound sleep or administration of the sleeping aid gaboxadol normalized BBB permeability, showing that SD effects on the BBB are reversible. Along with BBB permeability, RNA levels of the BBB master regulator moody are modulated by sleep. Conversely, altering BBB permeability alone through glia-specific modulation of moody, gαo, loco, lachesin, or neuroglian-each a well-studied regulator of BBB function-was sufficient to induce robust sleep phenotypes. These studies demonstrate a tight link between BBB permeability and sleep and indicate a unique role for the BBB in the regulation of sleep.
Keywords: GPCR; blood-brain-barrier; sleep.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Nichols A. L. A., Eichler T., Latham R., Zimmer M., A global brain state underlies C. elegans sleep behavior. Science 356, eaam6851 (2017). - PubMed
-
- Vaccaro A., et al. , Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 181, 1307–1328.e15 (2020). - PubMed
-
- Rechtschaffen A., Bergmann B. M., Sleep deprivation in the rat: An update of the 1989 paper. Sleep 25, 18–24 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

