Adverse and compensatory neurophysiological slowing in Parkinson's disease
- PMID: 37832713
- PMCID: PMC10872886
- DOI: 10.1016/j.pneurobio.2023.102538
Adverse and compensatory neurophysiological slowing in Parkinson's disease
Abstract
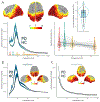
Patients with Parkinson's disease (PD) exhibit multifaceted changes in neurophysiological brain activity, hypothesized to represent a global cortical slowing effect. Using task-free magnetoencephalography and extensive clinical assessments, we found that neurophysiological slowing in PD is differentially associated with motor and non-motor symptoms along a sagittal gradient over the cortical anatomy. In superior parietal regions, neurophysiological slowing reflects an adverse effect and scales with cognitive and motor impairments, while across the inferior frontal cortex, neurophysiological slowing is compatible with a compensatory role. This adverse-to-compensatory gradient is sensitive to individual clinical profiles, such as drug regimens and laterality of symptoms; it is also aligned with the topography of neurotransmitter and transporter systems relevant to PD. We conclude that neurophysiological slowing in patients with PD signals both deleterious and protective mechanisms of the disease, from posterior to anterior regions across the cortex, respectively, with functional and clinical relevance to motor and cognitive symptoms.
Keywords: Functional gradient; Neurophysiological slowing; Parkinson’s disease; Spectral parameterization.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest none.
Figures

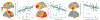


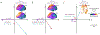

References
-
- Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, Weintraub D, 2021. Parkinson disease-associated cognitive impairment. Nature Reviews Disease Primers 7, 1–21. - PubMed
-
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57, 289–300.
-
- Berendse H, Verbunt J, Scheltens P, Van Dijk B, Jonkman E, 2000. Magnetoencephalographic analysis of cortical activity in Alzheimer’s disease: a pilot study. Clinical Neurophysiology 111, 604–612. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

