Perfluorooctanoic acid (PFOA) inhibits steroidogenesis and mitochondrial function in bovine granulosa cells in vitro
- PMID: 37832777
- PMCID: PMC10873118
- DOI: 10.1016/j.envpol.2023.122698
Perfluorooctanoic acid (PFOA) inhibits steroidogenesis and mitochondrial function in bovine granulosa cells in vitro
Abstract
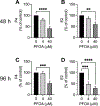
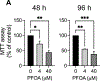
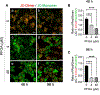
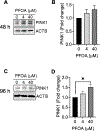
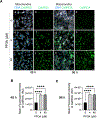
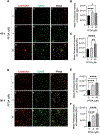
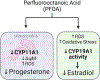
Perfluorooctanoic acid (PFOA) is a persistent environmental contaminant. Due to the ubiquitous presence of PFOA in the environment, the impacts of PFOA exposure not only affect human reproductive health but may also affect livestock reproductive health. The focus of this study was to determine the effects of PFOA on the physiological functions of bovine granulosa cells in vitro. Primary bovine granulosa cells were exposed to 0, 4, and 40 μM PFOA for 48 and 96 h followed by analysis of granulosa cell function including cell viability, steroidogenesis, and mitochondrial activity. Results revealed that PFOA inhibited steroid hormone secretion and altered the expression of key enzymes required for steroidogenesis. Gene expression analysis revealed decreases in mRNA transcripts for CYP11A1, HSD3B, and CYP19A1 and an increase in STAR expression after PFOA exposure. Similarly, PFOA decreased levels of CYP11A1 and CYP19A1 protein. PFOA did not impact live cell number, alter the cell cycle, or induce apoptosis, although it reduced metabolic activity, indicative of mitochondrial dysfunction. We observed that PFOA treatment caused a loss of mitochondrial membrane potential and increases in PINK protein expression, suggestive of mitophagy and mitochondrial damage. Further analysis revealed that these changes were associated with increased levels of reactive oxygen species. Expression of autophagy related proteins phosphoULK1 and LAMP2 were increased after PFOA exposure, in addition to an increased abundance of lysosomes, characteristic of increased autophagy. Taken together, these findings suggest that PFOA can negatively impact granulosa cell steroidogenesis via mitochondrial dysfunction.
Keywords: Perfluorooctanoic acid; autophagy; bovine; granulosa cells; mitochondria; steroidogenesis.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- BASINI G, BUSSOLATI S, TORCIANTI V & GRASSELLI F 2023. Perfluorooctanoic acid (PFOA) affects steroidogenesis and antioxidant defence in granulosa cells from swine ovary. Environ Toxicol Pharmacol, 101, 104169. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

