Neurodegenerative Disease Tauopathies
- PMID: 37832941
- PMCID: PMC11009985
- DOI: 10.1146/annurev-pathmechdis-051222-120750
Neurodegenerative Disease Tauopathies
Abstract
Tauopathies are a diverse group of progressive and fatal neurodegenerative diseases characterized by aberrant tau inclusions in the central nervous system. Tau protein forms pathologic fibrillar aggregates that are typically closely associated with neuronal cell death, leading to varied clinical phenotypes including dementia, movement disorders, and motor neuron disease. In this review, we describe the clinicopathologic features of tauopathies and highlight recent advances in understanding the mechanisms that lead to spread of pathologic aggregates through interconnected neuronal pathways. The cell-to-cell propagation of tauopathy is then linked to posttranslational modifications, tau fibril structural variants, and the breakdown of cellular protein quality control.
Keywords: AD; Alzheimer's disease; FTLD; MAPT; cryo-EM; cryogenic electron microscopy; frontotemporal lobar degeneration; microtubule-associated protein tau; proteostasis; tauopathy.
Conflict of interest statement
DISCLOSURE STATEMENT
E.B.L. and B.C.C. are listed as coinventors on a provisional patent titled “Small Molecule VCP Activators and Methods of Use.”
Figures
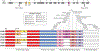

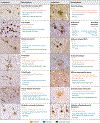

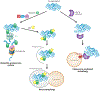
References
-
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. 2011. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70(11):960–69 - PubMed
-
- Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, et al. 2016. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann. Neurol 80(2):247–58 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

