Safety and Effectiveness of Regdanvimab for COVID-19 Treatment: A Phase 4 Post-marketing Surveillance Study Conducted in South Korea
- PMID: 37833467
- PMCID: PMC10600078
- DOI: 10.1007/s40121-023-00859-1
Safety and Effectiveness of Regdanvimab for COVID-19 Treatment: A Phase 4 Post-marketing Surveillance Study Conducted in South Korea
Abstract
Introduction: Regdanvimab, a neutralising monoclonal antibody (mAb) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), received approval for the treatment of coronavirus disease 2019 (COVID-19) in South Korea in 2021. The Ministry of Food and Drug Safety in South Korea mandate that new medications be re-examined for safety and effectiveness post-approval in at least 3000 individuals. This post-marketing surveillance (PMS) study was used to evaluate the safety and effectiveness of regdanvimab in real-world clinical care.
Methods: This prospective, multicentre, phase 4 PMS study was conducted between February 2021 and March 2022 in South Korea. Eligible patients were aged ≥ 18 years with confirmed mild COVID-19 at high risk of disease progression or moderate COVID-19. Patients were hospitalised and treated with regdanvimab (40 mg/kg, day 1) and then monitored until discharge, with a follow-up call on day 28. Adverse events (AEs) were documented, and the COVID-19 disease progression rate was used to measure effectiveness.
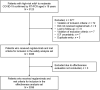
Results: Of the 3123 patients with COVID-19 infection identified, 3036 were eligible for inclusion. Approximately 80% and 5% of the eligible patients were diagnosed with COVID-19 during the delta- and omicron-dominant periods, respectively. Median (range) age was 57 (18-95) years, and 50.6% of patients were male. COVID-19 severity was assessed before treatment, and high-risk mild and moderate COVID-19 was diagnosed in 1030 (33.9%) and 2006 (66.1%) patients, respectively. AEs and adverse drug reactions (ADRs) were experienced by 684 (22.5%) and 363 (12.0%) patients, respectively. The most common ADR was increased liver function test (n = 62, 2.0%). Nine (0.3%) patients discontinued regdanvimab due to ADRs. Overall, 378 (12.5%) patients experienced disease progression after regdanvimab infusion, with extended hospitalisation/re-admission (n = 300, 9.9%) as the most common reason. Supplemental oxygen was required by 282 (9.3%) patients. Ten (0.3%) patients required intensive care monitoring and 3 (0.1%) died due to COVID-19.
Conclusion: This large-scale PMS study demonstrated that regdanvimab was effective against COVID-19 progression and had an acceptable safety profile when used in real-world clinical practice.
Keywords: COVID-19; CT-P59; Effectiveness; Monoclonal antibody; Neutralising antibody; Post-marketing surveillance; Regdanvimab; SARS-CoV-2; Safety.
© 2023. The Author(s).
Conflict of interest statement
Ji Yeon Lee has been an investigator on COVID-19 clinical trials sponsored by Daewoong Pharmaceuticals, Hyundai Bioscience, Shin Poong Pharmaceutical, and Ildong Pharmaceutical and on an investigator-initiated trial with Celltrion Inc. Seongcheol Cho has been an investigator on a COVID-19 clinical trial sponsored by Shin Poong Pharmaceutical. Jungok Kim and Sungmin Kym have been investigators on COVID-19 trials sponsored by Daewoong Pharmaceuticals, Hyundai Bioscience, Shin Poon Pharmaceutical, Ildong Pharmaceutical, ImmuneMed, Bioleaders, Dong-Wha, Pfizer, Gilead, and Celltrion Inc. Ki Tae Kwon has been an investigator on COVID-19 clinical trials sponsored by GC Holdings, Pfizer, Hyundai Bioscience, APRG Inc., Shin Poong Pharmaceutical, and Ildong Pharmaceutical and on an investigator-initiated trial with Celltrion Inc. Jin Yong Kim has been an investigator on COVID-19 clinical trials sponsored by Daewoong Pharmaceuticals, Enzychem Lifesciences, GC Pharma, Bukwang Pharmaceutical and Pfizer outside the scope of the submitted work, and on an investigator-initiated trial with Celltrion Inc. Sunghyun Kim, Keumyoung Ahn, Nahyun Jung, Yeonmi Lee, Yoobin Jung, and Chankyoung Hwang are employees of Celltrion Inc. Seon Hee Bu, EunHyang Song, Sungbong Yu, and Kwang Won Seo declare no interests related to this article. Sang Won Park has been an investigator on a COVID-19 clinical trial sponsored by Celltrion Inc. Seon Hee Bu was affiliated with Seoul Metropolitan City Bukbu Hospital at the time of the study, and is now affiliated with Kyung Hee University Hospital.
Figures
References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. 2023. https://www.who.int/europe/emergencies/situations/covid-19. Accessed 13 Apr 2023.
-
- World Health Organization. WHO coronavirus (COVID-19) dashboard. 2023. https://covid19.who.int/. Accessed 10 Mar 2023.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

