Caffeic acid alleviates cerebral ischemic injury in rats by resisting ferroptosis via Nrf2 signaling pathway
- PMID: 37833536
- PMCID: PMC10789749
- DOI: 10.1038/s41401-023-01177-5
Caffeic acid alleviates cerebral ischemic injury in rats by resisting ferroptosis via Nrf2 signaling pathway
Abstract
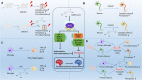
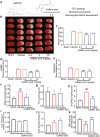
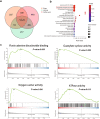
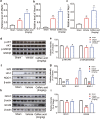
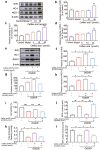
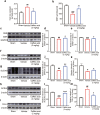
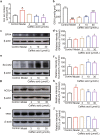
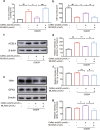
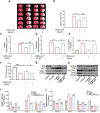
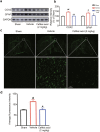
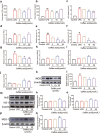
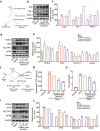
There are few effective and safe neuroprotective agents for the treatment of ischemic stroke currently. Caffeic acid is a phenolic acid that widely exists in a number of plant species. Previous studies show that caffeic acid ameliorates brain injury in rats after cerebral ischemia/reperfusion. In this study we explored the protective mechanisms of caffeic acid against oxidative stress and ferroptosis in permanent cerebral ischemia. Ischemia stroke was induced on rats by permanent middle cerebral artery occlusion (pMCAO). Caffeic acid (0.4, 2, 10 mg·kg-1·d-1, i.g.) was administered to the rats for 3 consecutive days before or after the surgery. We showed that either pre-pMCAO or post-pMCAO administration of caffeic acid (2 mg·kg-1·d-1) effectively reduced the infarct volume and improved neurological outcome. The therapeutic time window could last to 2 h after pMCAO. We found that caffeic acid administration significantly reduced oxidative damage as well as neuroinflammation, and enhanced antioxidant capacity in pMCAO rat brain. We further demonstrated that caffeic acid down-regulated TFR1 and ACSL4, and up-regulated glutathione production through Nrf2 signaling pathway to resist ferroptosis in pMCAO rat brain and in oxygen glucose deprivation/reoxygenation (OGD/R)-treated SK-N-SH cells in vitro. Application of ML385, an Nrf2 inhibitor, blocked the neuroprotective effects of caffeic acid in both in vivo and in vitro models, evidenced by excessive accumulation of iron ions and inactivation of the ferroptosis defense system. In conclusion, caffeic acid inhibits oxidative stress-mediated neuronal death in pMCAO rat brain by regulating ferroptosis via Nrf2 signaling pathway. Caffeic acid might serve as a potential treatment to relieve brain injury after cerebral ischemia. Caffeic acid significantly attenuated cerebral ischemic injury and resisted ferroptosis both in vivo and in vitro. The regulation of Nrf2 by caffeic acid initiated the transcription of downstream target genes, which were shown to be anti-inflammatory, antioxidative and antiferroptotic. The effects of caffeic acid on neuroinflammation and ferroptosis in cerebral ischemia were explored in a primary microglia-neuron coculture system. Caffeic acid played a role in reducing neuroinflammation and resisting ferroptosis through the Nrf2 signaling pathway, which further suggested that caffeic acid might be a potential therapeutic method for alleviating brain injury after cerebral ischemia.
Keywords: Nrf2; caffeic acid; ferroptosis; ischemic stroke; neuroinflammation; oxidative stress.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

