BNT162b2 vaccine induced variant-specific immunity, safety and risk of Omicron breakthrough infection in children aged 5 to 11 years: a cohort study
- PMID: 37833554
- PMCID: PMC10575958
- DOI: 10.1038/s41598-023-44565-x
BNT162b2 vaccine induced variant-specific immunity, safety and risk of Omicron breakthrough infection in children aged 5 to 11 years: a cohort study
Abstract
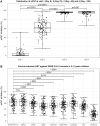
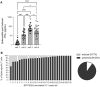
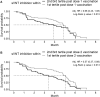
There is little information on BNT162b2 vaccine-induced variant-specific immunogenicity, safety data and dynamics of breakthrough infections in pediatric populations. We addressed these questions using a prospective two dose BNT162b2 (10 mcg) vaccination cohort study of healthy children 5-11 years in Singapore. Follow up included blood samples at scheduled visits, daily vaccination symptom diary and confirmation of SARS-CoV-2 infection. Surrogate virus neutralization test (sVNT) and spike-specific T cell responses against SARS-CoV-2 variants were performed. The mean age of 127 participants was 8.27 years (SD 1.95) and 51.2% were males. The median sVNT level against original variant after 1 dose and 2 dose vaccination was 61.4% and 95.1% respectively (p < 0.0001). Neutralizing antibodies against the Omicron variant was the lowest, median 22.4% (IQR 16.5-30.8). However, T cell IFN-γ cytokine response against Omicron variant was high and remained so about 4 months after vaccination. Fever rate increased significantly from 4% (dose 1) to 11.5% (dose 2). The risk of Omicron breakthrough infection decreased by 7.8% for every 1% increase in sVNT inhibition level measured after dose 2 vaccination. BNT162b2 vaccines were safe, induced good T cell responses but poor neutralizing antibodies against Omicron in children. Low neutralizing antibody levels post-vaccination was predictive of subsequent breakthrough infection.
© 2023. Springer Nature Limited.
Conflict of interest statement
CFY has received funding to attend conferences and honorarium from Sanofi, Pfizer and Takeda outside the current work. All other authors report nil conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

