The Role of Nrf2 and Inflammation on the Dissimilar Cardiotoxicity of Doxorubicin in Two-Time Points: a Cardio-Oncology In Vivo Study Through Time
- PMID: 37833616
- PMCID: PMC10799157
- DOI: 10.1007/s10753-023-01908-0
The Role of Nrf2 and Inflammation on the Dissimilar Cardiotoxicity of Doxorubicin in Two-Time Points: a Cardio-Oncology In Vivo Study Through Time
Abstract
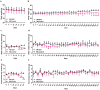
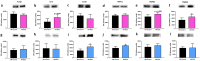
Doxorubicin (DOX) is a topoisomerase II inhibitor used in cancer therapy. Despite its efficacy, DOX causes serious adverse effects, such as short- and long-term cardiotoxicity. This work aimed to assess the short- and long-term cardiotoxicity of DOX and the role of inflammation and antioxidant defenses on that cardiotoxicity in a mice model. Adult CD-1 male mice received a cumulative dose of 9.0 mg/kg of DOX (2 biweekly intraperitoneal injections (ip), for 3 weeks). One week (1W) or 5 months (5M) after the last DOX administration, the heart was collected. One week after DOX, a significant increase in p62, tumor necrosis factor receptor (TNFR) 2, glutathione peroxidase 1, catalase, inducible nitric oxide synthase (iNOS) cardiac expression, and a trend towards an increase in interleukin (IL)-6, TNFR1, and B-cell lymphoma 2 associated X (Bax) expression was observed. Moreover, DOX induced a decrease on nuclear factor erythroid-2 related factor 2 (Nrf2) cardiac expression. In both 1W and 5M, DOX led to a high density of infiltrating M1 macrophages, but only the 1W-DOX group had a significantly higher number of nuclear factor κB (NF-κB) p65 immunopositive cells. As late effects (5M), an increase in Nrf2, myeloperoxidase, IL-33, tumor necrosis factor-α (TNF-α), superoxide dismutase 2 (SOD2) expression, and a trend towards increased catalase expression were observed. Moreover, B-cell lymphoma 2 (Bcl-2), cyclooxygenase-2 (COX-2), and carbonylated proteins expression decreased, and a trend towards decreased p38 mitogen-activated protein kinase (MAPK) expression were seen. Our study demonstrated that DOX induces adverse outcome pathways related to inflammation and oxidative stress, although activating different time-dependent response mechanisms.
Keywords: Nrf2.; cardiotoxicity; doxorubicin; inflammation; long-term effects; redox homeostasis disruption.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
- Ana Reis-Mendes and Vera Marisa Costa acknowledge FCT for their Grants: SFRH/ BD/129359/2017 and SFRH/BPD/110001/2015, respectively, being the latter funded by national funds through FCT-Fundação para a Ciência e a Tecnologia, I.P., under the Norma Transitória-DL57/2016/ CP1334/CT0006./FCT-Fundação para a Ciência e a Tecnologia, I.P
- This work was funded by national funds from FCT-Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences-UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy-i4HB./FCT-Fundação para a Ciência e a Tecnologia, I.P
LinkOut - more resources
Full Text Sources
Research Materials

