Murine sterile fecal filtrate is a potent pharmacological agent that exerts age-independent immunomodulatory effects in RAW264.7 macrophages
- PMID: 37833682
- PMCID: PMC10576334
- DOI: 10.1186/s12906-023-04193-4
Murine sterile fecal filtrate is a potent pharmacological agent that exerts age-independent immunomodulatory effects in RAW264.7 macrophages
Abstract
Background: Sterile fecal filtrate (SFF) is being considered a safer alternative to fecal microbiota transplantation (FMT) therapy; however, its bioactive potency is very little understood. The present study thus assessed the age-dependent immunostimulatory and immunomodulatory attributes of murine SFF in vitro.
Methods: SFF from young (Y-SFF) and old (O-SFF) Swiss albino mice were prepared. Immunostimulatory and immunomodulatory effects of SFF were evaluated in resting and lipopolysaccharide (LPS) stimulated macrophage cells by measuring intracellular reactive oxygen species (ROS), nitric oxide (NO) production, inflammatory cytokines profile, as well as gene expression of oxidative and inflammatory transcription factors. SFF were also evaluated for native antioxidant capacity by measuring DPPH and ABTS free radical scavenging activity. Bioactive components present in SFF were also determined by GC/MS analysis.
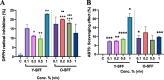
Results: Both Y-SFF and O-SFF induced potent immunostimulatory effects characterized by changes in cell morphology, a significant increase in NO production, ROS levels, and an increased ratio of pro-inflammatory (IL-6, TNF-α, IL-1β) to anti-inflammatory (IL-10) secretory proteins although no significant aggravation in the transcription of NF-κB and Nrf-2 could be observed. Application of LPS to cells significantly augmented a pro-oxidative and pro-inflammatory response which was much higher in comparison to Y-SFF or O-SFF application alone and mediated by strong suppression of Nrf-2 gene expression. Pre-treatment of macrophages with both Y-SFF and O-SFF robustly attenuated cellular hyperresponsiveness to LPS characterized by significantly decreased levels of NO, ROS, and inflammatory cytokines while a concomitant increase in anti-inflammatory protein (IL-10) was observed. Further, both Y-SFF and O-SFF strongly resisted LPS-induced downregulation of Nrf-2 expression although O-SFF appeared to protect cells slightly better from the overall LPS threat. Neat SFF samples exhibited moderate antioxidant capacity and GC/MS analysis of SFF revealed diverse volatile organic compounds characterized by alkanes, organosulphur compounds, furans, amides, amino acids, and antimicrobial elements.
Conclusion: Our results indicate that SFF is a potent stimulant of macrophages and confers strong anti-inflammatory effects regardless of donor age thereby suggesting its therapeutic efficacy in lieu of FMT therapy.
Keywords: Fecal microbiota transplantation; Inflammation; LPS; Macrophages; Sterile fecal filtrate.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Lu J, Ma KL, Ruan XZ. Dysbiosis of gut microbiota contributes to the development of diabetes mellitus. Infect Microbes Dis. 2019;1(2):43–48. doi: 10.1097/IM9.0000000000000011. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

