Neocortex neurogenesis and maturation in the African greater cane rat
- PMID: 37833718
- PMCID: PMC10571270
- DOI: 10.1186/s13064-023-00175-x
Neocortex neurogenesis and maturation in the African greater cane rat
Abstract
Background: Neocortex development has been extensively studied in altricial rodents such as mouse and rat. Identification of alternative animal models along the "altricial-precocial" spectrum in order to better model and understand neocortex development is warranted. The Greater cane rat (GCR, Thyronomys swinderianus) is an indigenous precocial African rodent. Although basic aspects of brain development in the GCR have been documented, detailed information on neocortex development including the occurrence and abundance of the distinct types of neural progenitor cells (NPCs) in the GCR are lacking.
Methods: GCR embryos and fetuses were obtained from timed pregnant dams between gestation days 50-140 and their neocortex was analyzed by immunofluorescence staining using characteristic marker proteins for NPCs, neurons and glia cells. Data were compared with existing data on closely related precocial and altricial species, i.e. guinea pig and dwarf rabbit.
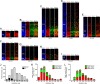
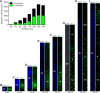
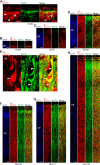
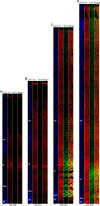
Results: The primary sequence of neuro- and gliogenesis, and neuronal maturation is preserved in the prenatal GCR neocortex. We show that the GCR exhibits a relatively long period of cortical neurogenesis of 70 days. The subventricular zone becomes the major NPC pool during mid-end stages of neurogenesis with Pax6 + NPCs constituting the major basal progenitor subtype in the GCR neocortex. Whereas dendrite formation in the GCR cortical plate appears to initiate immediately after the onset of neurogenesis, major aspects of axon formation and maturation, and astrogenesis do not begin until mid-neurogenesis. Similar to the guinea pig, the GCR neocortex exhibits a high maturation status, containing neurons with well-developed dendrites and myelinated axons and astrocytes at birth, thus providing further evidence for the notion that a great proportion of neocortex growth and maturation in precocial mammals occurs before birth.
Conclusions: Together, this work has deepened our understanding of neocortex development of the GCR, of the timing and the cellular differences that regulate brain growth and development within the altricial-precocial spectrum and its suitability as a research model for neurodevelopmental studies. The timelines of brain development provided by this study may serve as empirical reference data and foundation in future studies in order to model and better understand neurodevelopment and associated alterations.
Keywords: Altricial; Grasscutter; Greater cane rat; Neocortex development; Neurogenesis; Neuron maturation; Precocial.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Developmental Differences in Neocortex Neurogenesis and Maturation Between the Altricial Dwarf Rabbit and Precocial Guinea Pig.Front Neuroanat. 2021 May 31;15:678385. doi: 10.3389/fnana.2021.678385. eCollection 2021. Front Neuroanat. 2021. PMID: 34135738 Free PMC article.
-
Morphological Characterization of the Developing Greater Cane Rat (Thryonomys swinderianus) Brain.Dev Neurosci. 2020;42(2-4):114-123. doi: 10.1159/000510848. Epub 2020 Dec 15. Dev Neurosci. 2020. PMID: 33321497
-
Developmental horizons in the pre-natal development of the Greater cane rat (Thryonomys swinderianus).Anat Histol Embryol. 2019 Sep;48(5):486-497. doi: 10.1111/ahe.12470. Epub 2019 Jul 17. Anat Histol Embryol. 2019. PMID: 31314146
-
How neural stem cells contribute to neocortex development.Biochem Soc Trans. 2021 Nov 1;49(5):1997-2006. doi: 10.1042/BST20200923. Biochem Soc Trans. 2021. PMID: 34397081 Free PMC article. Review.
-
A study of scientific publications on the greater cane rat (Thryonomys swinderianus, Temminck 1827).Animal Model Exp Med. 2020 Mar 22;3(1):40-46. doi: 10.1002/ame2.12103. eCollection 2020 Mar. Animal Model Exp Med. 2020. PMID: 32318658 Free PMC article. Review.
References
-
- Aluko FA, Salako AE, Ngere LO, Awojobi HA. Reproductive history of cane rat: a review of the reproduction and reproductive performance. J Agric Soc Res. 2014;14(1):109–115.
MeSH terms
LinkOut - more resources
Full Text Sources

