Effects of autologous serum on TREM2 and APOE in a personalized monocyte-derived macrophage assay of late-onset Alzheimer's patients
- PMID: 37833781
- PMCID: PMC10576307
- DOI: 10.1186/s12979-023-00376-2
Effects of autologous serum on TREM2 and APOE in a personalized monocyte-derived macrophage assay of late-onset Alzheimer's patients
Abstract
Background: Age-associated deterioration of the immune system contributes to a chronic low-grade inflammatory state known as "inflammaging" and is implicated in the pathogenesis of late-onset Alzheimer's disease (LOAD). Whether changes in the tissue environment caused by circulatory factors associated with aging may alter the innate immune response is unknown. Monocyte-derived macrophages (Mo-MФs) infiltrating the brain alongside microglia are postulated to play a modulatory role in LOAD and both express triggering receptor expressed on myeloid cells 2 (TREM2). Apolipoprotein E (APOE) acts as a ligand for TREM2, and their role in amyloid beta (Aβ) clearance highlights their importance in LOAD. However, the influence of the patient's own milieu (autologous serum) on the synthesis of TREM2 and APOE in infiltrating macrophages remains unknown.
Objectives: To functionally assess patient-specific TREM2 and APOE synthesis, we designed a personalized assay based on Mo-MФs using monocytes from LOAD patients and matched controls (CO). We assessed the influence of each participant's own milieu, by examining the effect of short- (1 day) and long- (10 days) term differentiation of the cells in the presence of the donor´s autologous serum (AS) into M1-, M2- or M0-macrophages. Additionally, sex differences and Aβ-uptake ability in short- and long-term differentiated Mo-MФs were assessed.
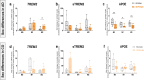
Results: We showed a time-dependent increase in TREM2 and APOE protein levels in LOAD- and CO-derived cells. While AS did not differentially modulate TREM2 compared to standard fetal calf serum (FCS), AS decreased APOE levels in M2 macrophages but increased levels in M1 macrophages. Interestingly, higher levels of TREM2 and lower levels of APOE were detected in female- than in male- LOAD patients. Finally, we report decreased Aβ-uptake in long-term differentiated CO- and LOAD-derived cells, particularly in APOEε4(+) carriers.
Conclusions: We demonstrate for the first time the suitability of a personalized Mo-MФ cell culture-based assay for studying functional TREM2 and APOE synthesis in a patient's own aged milieu. Our strategy may thus provide a useful tool for future research on diagnostic and therapeutic aspects of personalized medicine.
Keywords: APOE; Amyloid-beta uptake; Inflammaging; Late-onset Alzheimer's disease; Monocyte-derived macrophages; Patient-derived personalized assay; Sex differences; TREM2.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Prince M. World Alzheimer Report 2015: The Global Impact of Dementia | Alzheimer’s Disease International. World Alzheimer’s Report. 2015.
-
- Sheppard O., & Coleman M. Alzheimer’s Disease: Etiology, Neuropathology and Pathogenesis. Alzheimer’s Disease: Drug Discovery. 2020:1–22. 10.36255/EXONPUBLICATIONS.ALZHEIMERSDISEASE.2020.CH1.
-
- Talwar P., Sinha J., Grover S., Rawat C., Kushwaha S., Agarwal R., Taneja V., & Kukreti R. Dissecting Complex and Multifactorial Nature of Alzheimer’s Disease Pathogenesis: a Clinical, Genomic, and Systems Biology Perspective. Molecular Neurobiology 2015 ;53(7): 4833–4864. 10.1007/S12035-015-9390-0. - PubMed
-
- Heneka M. T., O’Banion M. K., Terwel D., & Kummer M. P. Neuroinflammatory processes in Alzheimer’s disease. J Neural Trans 2010;117(8):117(8):919–947. 10.1007/S00702-010-0438-Z. - PubMed
-
- Regen F., Hellmann-Regen J., Costantini E., & Reale M. Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Current Alzheimer Research. 2017;14(11). 10.2174/1567205014666170203141717. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

