Testosterone upregulates glial cell line-derived neurotrophic factor (GDNF) and promotes neuroinflammation to enhance glioma cell survival and proliferation
- PMID: 37833789
- PMCID: PMC10571473
- DOI: 10.1186/s41232-023-00300-7
Testosterone upregulates glial cell line-derived neurotrophic factor (GDNF) and promotes neuroinflammation to enhance glioma cell survival and proliferation
Abstract
Background: Testosterone contributes to male organism development, such as bone density, muscle development, and fat repartition. Estrogen (derived from testosterone) also contributes to female reproductive system development. Here, we investigated the effect of testosterone on glioma cells and brain neuron inflammation essential for cancer development and progression.
Methods: The human astrocyte and glioma cell lines were treated with 6 ng/ml exogenous testosterone in vitro. We performed cell counting kit-8, transwell, and wound healing assays to determine the effect of testosterone on glioma cell proliferation, migration, and invasion. The glioma cells were injected into the xenograft and treated with 5 µl concentrated testosterone. Transcriptional suppression of glial cell line-derived neurotrophic factor (GDNF) was performed to evaluate brain neuron inflammation and survival. The tumor tissues were assessed by hematoxylin-eosin staining and immunohistochemistry.
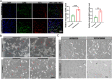
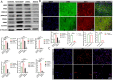
Results: Testosterone upregulates GDNF to stimulate proliferation, migration, and invasion of glioma cells. Pathologically, the augmentation of GDNF and cyclophilin A contributed to neuroprotection when treated with testosterone. Our investigation showed that testosterone contributes to brain neuron and astrocyte inflammation through the upregulation of nuclear factor erythroid 2-related factor 2 (NRF2), glial fibrillary acid protein (GFAP), and sirtuin 5 (SIRT5), resulting in pro-inflammatory macrophages recruitments into the neural microenvironment. Mechanically, testosterone treatment regulates GDNF translocation from the glioma cells and astrocyte nuclei to the cytoplasm.
Conclusion: Testosterone upregulates GDNF in glioma cells and astrocytes essential for microglial proliferation, migration, and invasion. Testosterone contributes to brain tumor growth via GDNF and inflammation. The contribution of testosterone, macrophages, and astrocytes, in old neuron rescue, survival, and proliferation. During brain neuron inflammation, the organism activates and stimulates the neuron rescue through the enrichment of the old neuron microenvironment with growth factors such as GDNF, BDNF, SOX1/2, and MAPK secreted by the surrounding neurons and glial cells to maintain the damaged neuron by inflammation alive even if the axon is dead. The immune response also contributes to brain cell survival through the secretion of proinflammatory cytokines, resulting in inflammation maintenance. The rescued old neuron interaction with infiltrated macrophages contributes to angiogenesis to supplement the old neuron with more nutrients leading to metabolism activation and surrounding cell uncontrollable cell growth.
Keywords: Cyclophilin A; GDNF; Glioma; Neuroinflammation; Neuroprotection; Testosterone.
© 2023. Japanese Society of Inflammation and Regeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Jakob S, Lovell-Badge R. Sex determination and the control of Sox9 expression in mammals. FEBS J. 2011;278(7):1002–1009. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

