Enhanced Antibacterial Ability of Electrospun PCL Scaffolds Incorporating ZnO Nanowires
- PMID: 37833866
- PMCID: PMC10572921
- DOI: 10.3390/ijms241914420
Enhanced Antibacterial Ability of Electrospun PCL Scaffolds Incorporating ZnO Nanowires
Abstract
The infection of implanted biomaterial scaffolds presents a major challenge. Existing therapeutic solutions, such as antibiotic treatment and silver nanoparticle-containing scaffolds are becoming increasingly impractical because of the growth of antibiotic resistance and the toxicity of silver nanoparticles. We present here a novel concept to overcome these limitations, an electrospun polycaprolactone (PCL) scaffold functionalised with zinc oxide nanowires (ZnO NWs). This study assessed the antibacterial capabilities and biocompatibility of PCL/ZnO scaffolds. The fabricated scaffolds were characterised by SEM and EDX, which showed that the ZnO NWs were successfully incorporated and distributed in the electrospun PCL scaffolds. The antibacterial properties were investigated by co-culturing PCL/ZnO scaffolds with Staphylococcus aureus. Bacterial colonisation was reduced to 51.3% compared to a PCL-only scaffold. The biocompatibility of the PCL/ZnO scaffolds was assessed by culturing them with HaCaT cells. The PCL scaffolds exhibited no changes in cell metabolic activity with the addition of the ZnO nanowires. The antibacterial and biocompatibility properties make PCL/ZnO a good choice for implanted scaffolds, and this work lays a foundation for ZnO NWs-infused PCL scaffolds in the potential clinical application of tissue engineering.
Keywords: PCL scaffold; ZnO nanowire; antimicrobial; electrospinning; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


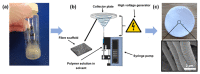


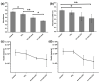

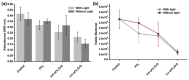
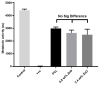
References
-
- Qian Y., Zhou X., Zhang F., Diekwisch T.G., Luan X., Yang J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces. 2019;11:37381–37396. doi: 10.1021/acsami.9b07053. - DOI - PMC - PubMed
-
- Ahmed M.K., Zayed M.A., El-Dek S.I., Hady M.A., El Sherbiny D.H., Uskokovic V. Nanofibrous epsilon-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: Physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo. Bioact. Mater. 2021;6:2070–2088. doi: 10.1016/j.bioactmat.2020.12.026. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

