Exploring Tumor-Immune Interactions in Co-Culture Models of T Cells and Tumor Organoids Derived from Patients
- PMID: 37834057
- PMCID: PMC10572813
- DOI: 10.3390/ijms241914609
Exploring Tumor-Immune Interactions in Co-Culture Models of T Cells and Tumor Organoids Derived from Patients
Abstract
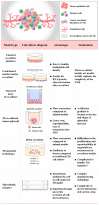
The use of patient-derived tumor tissues and cells has led to significant advances in personalized cancer therapy and precision medicine. The advent of genomic sequencing technologies has enabled the comprehensive analysis of tumor characteristics. The three-dimensional tumor organoids derived from self-organizing cancer stem cells are valuable ex vivo models that faithfully replicate the structure, unique features, and genetic characteristics of tumors. These tumor organoids have emerged as innovative tools that are extensively employed in drug testing, genome editing, and transplantation to guide personalized therapy in clinical settings. However, a major limitation of this emerging technology is the absence of a tumor microenvironment that includes immune and stromal cells. The therapeutic efficacy of immune checkpoint inhibitors has underscored the importance of immune cells, particularly cytotoxic T cells that infiltrate the vicinity of tumors, in patient prognosis. To address this limitation, co-culture techniques combining tumor organoids and T cells have been developed, offering diverse avenues for studying individualized drug responsiveness. By integrating cellular components of the tumor microenvironment, including T cells, into tumor organoid cultures, immuno-oncology has embraced this technology, which is rapidly advancing. Recent progress in co-culture models of tumor organoids has allowed for a better understanding of the advantages and limitations of this novel model, thereby exploring its full potential. This review focuses on the current applications of organoid-T cell co-culture models in cancer research and highlights the remaining challenges that need to be addressed for its broader implementation in anti-cancer therapy.
Keywords: T cells; co-culture system; patient-derived organoid; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Giraldo N.A., Taube J.M. In: PD-L1 and Other Immunological Diagnosis Tools. Zitvogel L., Kroemer G., editors. Springer; Baltimore, MD, USA: 2018. pp. 371–385.
-
- Hammers H.J., Plimack E.R., Infante J.R., Rini B.I., McDermott D.F., Lewis L.D., Voss M.H., Sharma P., Pal S.K., Razak A.R.A., et al. Safety and Efficacy of Nivolumab in Combination with Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J. Clin. Oncol. 2017;35:3851–3858. doi: 10.1200/JCO.2016.72.1985. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

