NET Formation Was Reduced via Exposure to Extremely Low-Frequency Pulsed Electromagnetic Fields
- PMID: 37834077
- PMCID: PMC10572227
- DOI: 10.3390/ijms241914629
NET Formation Was Reduced via Exposure to Extremely Low-Frequency Pulsed Electromagnetic Fields
Abstract
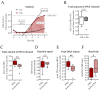
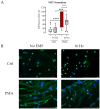
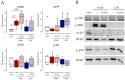
Fracture-healing is a highly complex and timely orchestrated process. Non-healing fractures are still a major clinical problem and treatment remains difficult. A 16 Hz extremely low-frequency pulsed electromagnetic field (ELF-PEMF) was identified as non-invasive adjunct therapy supporting bone-healing by inducing reactive oxygen species (ROS) and Ca2+-influx. However, ROS and Ca2+-influx may stimulate neutrophils, the first cells arriving at the wounded site, to excessively form neutrophil extracellular traps (NETs), which negatively affects the healing process. Thus, this study aimed to evaluate the effect of this 16 Hz ELF-PEMF on NET formation. Neutrophils were isolated from healthy volunteers and exposed to different NET-stimuli and the 16 Hz ELF-PEMF. NETs were quantified using Sytox Green Assay and immunofluorescence, Ca2+-influx and ROS with fluorescence probes. In contrast to mesenchymal cells, ELF-PEMF exposure did not induce ROS and Ca2+-influx in neutrophils. ELF-PEMF exposure did not result in basal or enhanced PMA-induced NET formation but did reduce the amount of DNA released. Similarly, NET formation induced by LPS and H2O2 was reduced through exposure to ELF-PEMF. As ELF-PEMF exposure did not induce NET release or negatively affect neutrophils, the ELF-PEMF exposure can be started immediately after fracture treatment.
Keywords: EMF; fracture healing; neutrophil extracellular traps; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest. Sachtleben GmbH provided the ELF-PEMF devices with the technical support but was not involved in the study design or the data evaluation.
Figures


References
-
- Ziegler P., Nussler A.K., Wilbrand B., Falldorf K., Springer F., Fentz A.K., Eschenburg G., Ziegler A., Stöckle U., Maurer E., et al. Pulsed Electromagnetic Field Therapy Improves Osseous Consolidation after High Tibial Osteotomy in Elderly Patients-A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Med. 2019;8:2008. doi: 10.3390/jcm8112008. - DOI - PMC - PubMed
-
- Caliogna L., Medetti M., Bina V., Brancato A.M., Castelli A., Jannelli E., Ivone A., Gastaldi G., Annunziata S., Mosconi M., et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021;22:7403. doi: 10.3390/ijms22147403. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

