Iron Oxide Nanoparticles with Supramolecular Ureido-Pyrimidinone Coating for Antimicrobial Peptide Delivery
- PMID: 37834098
- PMCID: PMC10573039
- DOI: 10.3390/ijms241914649
Iron Oxide Nanoparticles with Supramolecular Ureido-Pyrimidinone Coating for Antimicrobial Peptide Delivery
Abstract
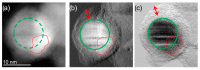
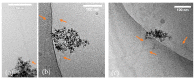
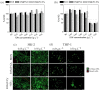
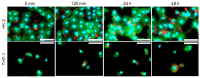
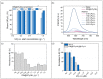
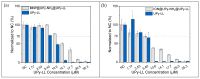
Antimicrobial peptides (AMPs) can kill bacteria by disrupting their cytoplasmic membrane, which reduces the tendency of antibacterial resistance compared to conventional antibiotics. Their possible toxicity to human cells, however, limits their applicability. The combination of magnetically controlled drug delivery and supramolecular engineering can help to reduce the dosage of AMPs, control the delivery, and improve their cytocompatibility. Lasioglossin III (LL) is a natural AMP form bee venom that is highly antimicrobial. Here, superparamagnetic iron oxide nanoparticles (IONs) with a supramolecular ureido-pyrimidinone (UPy) coating were investigated as a drug carrier for LL for a controlled delivery to a specific target. Binding to IONs can improve the antimicrobial activity of the peptide. Different transmission electron microscopy (TEM) techniques showed that the particles have a crystalline iron oxide core with a UPy shell and UPy fibers. Cytocompatibility and internalization experiments were carried out with two different cell types, phagocytic and nonphagocytic cells. The drug carrier system showed good cytocompatibility (>70%) with human kidney cells (HK-2) and concentration-dependent toxicity to macrophagic cells (THP-1). The particles were internalized by both cell types, giving them the potential for effective delivery of AMPs into mammalian cells. By self-assembly, the UPy-coated nanoparticles can bind UPy-functionalized LL (UPy-LL) highly efficiently (99%), leading to a drug loading of 0.68 g g-1. The binding of UPy-LL on the supramolecular nanoparticle system increased its antimicrobial activity against E. coli (MIC 3.53 µM to 1.77 µM) and improved its cytocompatible dosage for HK-2 cells from 5.40 µM to 10.6 µM. The system showed higher cytotoxicity (5.4 µM) to the macrophages. The high drug loading, efficient binding, enhanced antimicrobial behavior, and reduced cytotoxicity makes ION@UPy-NH2 an interesting drug carrier for AMPs. The combination with superparamagnetic IONs allows potential magnetically controlled drug delivery and reduced drug amount of the system to address intracellular infections or improve cancer treatment.
Keywords: antimicrobial peptide; cytocompatibility; intracellular delivery; iron oxide nanoparticles; supramolecular system; ureido-pyrimidinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bryson V., Szybalski W. Microbial drug resistance. Adv. Genet. 1955;7:1–46. - PubMed
-
- United Nations Sustainable Development Goals: Take Action for the Sustainable Development Goals. [(accessed on 22 November 2022)]. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

