The Effect of Polymer Blends on the In Vitro Release/Degradation and Pharmacokinetics of Moxidectin-Loaded PLGA Microspheres
- PMID: 37834176
- PMCID: PMC10573114
- DOI: 10.3390/ijms241914729
The Effect of Polymer Blends on the In Vitro Release/Degradation and Pharmacokinetics of Moxidectin-Loaded PLGA Microspheres
Abstract
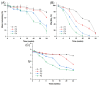
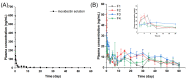
To investigate the effect of polymer blends on the in vitro release/degradation and pharmacokinetics of moxidectin-loaded PLGA microspheres (MOX-MS), four formulations (F1, F2, F3 and F4) were prepared using the O/W emulsion solvent evaporation method by blending high (75/25, 75 kDa) and low (50/50, 23 kDa) molecular weight PLGA with different ratios. The addition of low-molecular-weight PLGA did not change the release mechanism of microspheres, but sped up the drug release of microspheres and drastically shortened the lag phase. The in vitro degradation results show that the release of microspheres consisted of a combination of pore diffusion and erosion, and especially autocatalysis played an important role in this process. Furthermore, an accelerated release method was also developed to reduce the period for drug release testing within one month. The pharmacokinetic results demonstrated that MOX-MS could be released for at least 60 days with only a slight blood drug concentration fluctuation. In particular, F3 displayed the highest AUC and plasma concentration (AUC0-t = 596.53 ng/mL·d, Cave (day 30-day 60) = 8.84 ng/mL), making it the optimal formulation. Overall, these results indicate that using polymer blends could easily adjust hydrophobic drug release from microspheres and notably reduce the lag phase of microspheres.
Keywords: PLGA microspheres; in vitro release; moxidectin; pharmacokinetics; polymer blends.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
- 2021CG0024-02/the Special project for transformation of scientific and technological achievements in Inner Mongolia Autonomous Region of China under Grant
- 20YF8NA028/Key Research and Development Program of Gansu Province under Grant
- No. 25-LZIHPS-03/the Chinese Academy of Agricultural Sciences Innovative Project Veterinary Natural Medicine under Grant
- No. 1610032021014/Lanzhou Institute of Animal Husbandry and Veterinary Medicine of CAAS Fundamental Research Funds under Grant
LinkOut - more resources
Full Text Sources
Miscellaneous

