Aβ1-42 Accumulation Accompanies Changed Expression of Ly6/uPAR Proteins, Dysregulation of the Cholinergic System, and Degeneration of Astrocytes in the Cerebellum of Mouse Model of Early Alzheimer Disease
- PMID: 37834299
- PMCID: PMC10573428
- DOI: 10.3390/ijms241914852
Aβ1-42 Accumulation Accompanies Changed Expression of Ly6/uPAR Proteins, Dysregulation of the Cholinergic System, and Degeneration of Astrocytes in the Cerebellum of Mouse Model of Early Alzheimer Disease
Abstract
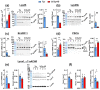
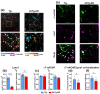
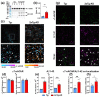
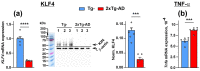
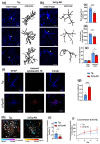
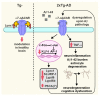
Alzheimer disease (AD) is a widespread neurodegenerative disease characterized by the accumulation of oligomeric toxic forms of β-amyloid (Aβ1-42) and dysfunction of the cholinergic system in the different brain regions. However, the exact mechanisms of AD pathogenesis and the role of the nicotinic acetylcholine receptors (nAChRs) in the disease progression remain unclear. Here, we revealed a decreased expression of a number of the Ly6/uPAR proteins targeting nAChRs in the cerebellum of 2xTg-AD mice (model of early AD) in comparison with non-transgenic mice both at mRNA and protein levels. We showed that co-localization of one of them, - neuromodulator Lynx1, with α7-nAChR was diminished in the vicinity of cerebellar astrocytes of 2xTg-AD mice, while Aβ1-42 co-localization with this receptor present was increased. Moreover, the expression of anti-inflammatory transcription factor KLF4 regulating transcription of the Ly6/uPAR genes was decreased in the cerebellum of 2xTg-AD mice, while expression of inflammatory cytokine TNF-α was increased. Based on these data together with observed astrocyte degeneration in the cerebellum of 2xTg-AD mice, we suggest the mechanism by which expression of the Ly6/uPAR proteins upon Aβ pathology results in dysregulation of the cholinergic system and particularly of α7-nAChR function in the cerebellum. This leads to enhanced neuroinflammation and cerebellar astrocyte degeneration.
Keywords: Alzheimer disease; Ly6/uPAR; Lynx1; Lypd6; PSCA; SLURP-1; astrocytes; motor memory; nicotinic acetylcholine receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Global Action Plan on the Public Health Response to Dementia 2017–2025. [(accessed on 1 November 2022)]. Available online: https://www.who.int/publications-detail-redirect/9789241513487.
-
- Liang C.-S., Li D.-J., Yang F.-C., Tseng P.-T., Carvalho A.F., Stubbs B., Thompson T., Mueller C., Shin J.I., Radua J., et al. Mortality Rates in Alzheimer’s Disease and Non-Alzheimer’s Dementias: A Systematic Review and Meta-Analysis. Lancet Healthy Longev. 2021;2:e479–e488. doi: 10.1016/S2666-7568(21)00140-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

