Structural and Electromagnetic Signatures of Anatase and Rutile NTs and Sheets in Three Different Water Models under Different Temperature Conditions
- PMID: 37834327
- PMCID: PMC10573416
- DOI: 10.3390/ijms241914878
Structural and Electromagnetic Signatures of Anatase and Rutile NTs and Sheets in Three Different Water Models under Different Temperature Conditions
Abstract
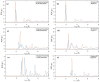
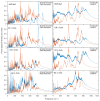
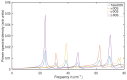
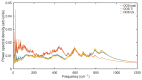
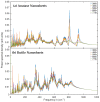
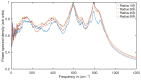
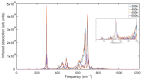
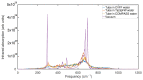
Experimental studies of TiO2 nanotubes have been conducted for nearly three decades and have revealed the remarkable advantages of this material. Research based on computer simulations is much rarer, with research using density functional theory (DFT) being the most significant in this field. It should be noted, however, that this approach has significant limitations when studying the macroscopic properties of nanostructures such as nanosheets and nanotubes. An alternative with great potential has emerged: classical molecular dynamics simulations (MD). MD Simulations offer the possibility to study macroscopic properties such as the density of phonon states (PDOS), power spectra, infrared spectrum, water absorption and others. From this point of view, the present study focuses on the distinction between the phases of anatase and rutile TiO2. The LAMMPS package is used to study both the structural properties by applying the radial distribution function (RDF) and the electromagnetic properties of these phases. Our efforts are focused on exploring the effect of temperature on the vibrational properties of TiO2 anatase nanotubes and an in-depth analysis of how the phononic softening phenomenon affects TiO2 nanostructures to improve the fundamental understanding in different dimensions and morphological configurations. A careful evaluation of the stability of TiO2 nanolamines and nanotubes at different temperatures is performed, as well as the adsorption of water on the nanosurface of TiO2, using three different water models.
Keywords: LAMMPS MD; Matsui and Akaogi; PDOS; antase; infrared spectra; low frequency; nanosheets; nanotubes; power spectra; radial distribution function (RDF); rutile; soft phonon effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Almomani M., Ahmed N., Rashid M., Ibnaouf K., Aldaghri O., Madkhali N., Cabrera H. Performance improvement of graded bandgap solar cell via optimization of energy levels alignment in Si quantum dot, TiO2 nanoparticles, and porous Si. Photonics. 2022;9:843. doi: 10.3390/photonics9110843. - DOI
-
- Moma J., Baloyi J. Photocatalysts: Applications and Attributes. Volume 18 BoD—Books on Demand; Norderstedt, Germany: 2019. Modified titanium dioxide for photocatalytic applications.
-
- Kılınç N., Şennik E., Işık M., Ahsen A., Öztürk O., Öztürk Z. Fabrication and gas sensing properties of C-doped and un-doped TiO2 nanotubes. Ceram. Int. 2014;40:109–115. doi: 10.1016/j.ceramint.2013.05.110. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

