Lp(a) in the Pathogenesis of Aortic Stenosis and Approach to Therapy with Antisense Oligonucleotides or Short Interfering RNA
- PMID: 37834387
- PMCID: PMC10573862
- DOI: 10.3390/ijms241914939
Lp(a) in the Pathogenesis of Aortic Stenosis and Approach to Therapy with Antisense Oligonucleotides or Short Interfering RNA
Abstract
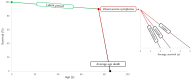
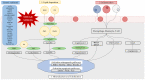
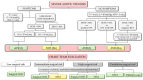
To date, no medical therapy can slow the progression of aortic stenosis. Fibrocalcific stenosis is the most frequent form in the general population and affects about 6% of the elderly population. Over the years, diagnosis has evolved thanks to echocardiography and computed tomography assessments. The application of artificial intelligence to electrocardiography could further implement early diagnosis. Patients with severe aortic stenosis, especially symptomatic patients, have valve repair as their only therapeutic option by surgical or percutaneous technique (TAVI). The discovery that the pathogenetic mechanism of aortic stenosis is similar to the atherosclerosis process has made it possible to evaluate the hypothesis of medical therapy for aortic stenosis. Several drugs have been tested to reduce low-density lipoprotein (LDL) and lipoprotein(a) (Lp(a)) levels, inflammation, and calcification. The Proprotein Convertase Subtilisin/Kexin type 9 inhibitors (PCSK9-i) could decrease the progression of aortic stenosis and the requirement for valve implantation. Great interest is related to circulating Lp(a) levels as causally linked to degenerative aortic stenosis. New therapies with ASO (antisense oligonucleotides) and siRNA (small interfering RNA) are currently being tested. Olpasiran and pelacarsen reduce circulating Lp(a) levels by 85-90%. Phase 3 studies are underway to evaluate the effect of these drugs on cardiovascular events (cardiovascular death, non-fatal myocardial injury, and non-fatal stroke) in patients with elevated Lp(a) and CVD (cardiovascular diseases). For instance, if a reduction in Lp(a) levels is associated with aortic stenosis prevention or progression, further prospective clinical trials are warranted to confirm this observation in this high-risk population.
Keywords: AVR; Lp(a); TAVI; aortic valve stenosis; artificial intelligence; calcification; dyslipidemia; inflammation; olpasiran; pelacarsen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yutzey K.E., Demer L.L., Body S.C., Huggins G.S., Towler D.A., Giachelli C.M., Hofmann-Bowman M.A., Mortlock D.P., Rogers M.B., Sadeghi M.M., et al. Calcific Aortic Valve Disease: A Consensus Summary from the Alliance of Investigators on Calcific Aortic Valve Disease. ATVB. 2014;34:2387–2393. doi: 10.1161/ATVBAHA.114.302523. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

