Diaphragm Fatigue in SMNΔ7 Mice and Its Molecular Determinants: An Underestimated Issue
- PMID: 37834400
- PMCID: PMC10574014
- DOI: 10.3390/ijms241914953
Diaphragm Fatigue in SMNΔ7 Mice and Its Molecular Determinants: An Underestimated Issue
Abstract
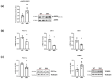
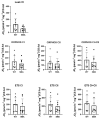
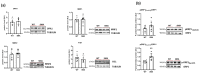
Spinal muscular atrophy (SMA) is a genetic disorder characterized by the loss of spinal motor neurons leading to muscle weakness and respiratory failure. Mitochondrial dysfunctions are found in the skeletal muscle of patients with SMA. For obvious ethical reasons, the diaphragm muscle is poorly studied, notwithstanding the very important role that respiratory involvement plays in SMA mortality. The main goal of this study was to investigate diaphragm functionality and the underlying molecular adaptations in SMNΔ7 mice, a mouse model that exhibits symptoms similar to that of patients with intermediate type II SMA. Functional, biochemical, and molecular analyses on isolated diaphragm were performed. The obtained results suggest the presence of an intrinsic energetic imbalance associated with mitochondrial dysfunction and a significant accumulation of reactive oxygen species (ROS). In turn, ROS accumulation can affect muscle fatigue, cause diaphragm wasting, and, in the long run, respiratory failure in SMNΔ7 mice. Exposure to the antioxidant molecule ergothioneine leads to the functional recovery of the diaphragm, confirming the presence of mitochondrial impairment and redox imbalance. These findings suggest the possibility of carrying out a dietary supplementation in SMNΔ7 mice to preserve their diaphragm function and increase their lifespan.
Keywords: SMA; diaphragm muscle; ergothioneine; muscle fatigue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

