The Impact of COVID-19 on Cellular Factors Influencing Red Blood Cell Aggregation Examined in Dextran: Possible Causes and Consequences
- PMID: 37834401
- PMCID: PMC10573242
- DOI: 10.3390/ijms241914952
The Impact of COVID-19 on Cellular Factors Influencing Red Blood Cell Aggregation Examined in Dextran: Possible Causes and Consequences
Abstract
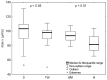
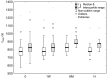
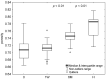
Several studies have indicated that COVID-19 can lead to alterations in blood rheology, including an increase in red blood cell aggregation. The precise mechanisms behind this phenomenon are not yet fully comprehended. The latest findings suggest that erythrocyte aggregation significantly influences microcirculation, causes the formation of blood clots in blood vessels, and even damages the endothelial glycocalyx, leading to endothelial dysfunction. The focus of this research lies in investigating the cellular factors influencing these changes in aggregation and discussing potential causes and implications in the context of COVID-19 pathophysiology. For this purpose, the aggregation of erythrocytes in a group of 52 patients with COVID-19 pneumonia was examined in a 70 kDa Dextran solution, which eliminates the influence of plasma factors. Using image analysis, the velocities and sizes of the formed aggregates were investigated, determining their porosity. This study showed that the process of erythrocyte aggregation in COVID-19 patients, independent of plasma factors, leads to the formation of more compact, denser, three-dimensional aggregates. These aggregates may be less likely to disperse under circulatory shear stress, increasing the risk of thrombotic events. This study also suggests that cellular aggregation factors can be responsible for the thrombotic disorders observed long after infection, even when plasma factors have normalized. The results and subsequent broad discussion presented in this study can contribute to a better understanding of the potential complications associated with increased erythrocyte aggregation.
Keywords: COVID-19; blood rheology; pneumonia; porosity of aggregates; red blood cells aggregation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Relationship between red blood cell aggregation and dextran molecular mass.Sci Rep. 2022 Nov 17;12(1):19751. doi: 10.1038/s41598-022-24166-w. Sci Rep. 2022. PMID: 36396711 Free PMC article.
-
Simultaneous influence of erythrocyte deformability and macromolecules in the medium on erythrocyte aggregation: a kinetic study by a laser scattering technique.Biochim Biophys Acta. 1994 Sep 14;1194(2):255-63. doi: 10.1016/0005-2736(94)90307-7. Biochim Biophys Acta. 1994. PMID: 7522564
-
Cell-cell affinity of senescent human erythrocytes.Biophys J. 2003 Jul;85(1):75-84. doi: 10.1016/S0006-3495(03)74456-7. Biophys J. 2003. PMID: 12829466 Free PMC article.
-
Rheology of the cerebral circulation.Neurosurgery. 1984 Jul;15(1):125-31. doi: 10.1227/00006123-198407000-00026. Neurosurgery. 1984. PMID: 6206438 Review.
-
[Red blood cell aggredation: mechanisms, adrenergic regulation].Ross Fiziol Zh Im I M Sechenova. 2007 Dec;93(12):1382-93. Ross Fiziol Zh Im I M Sechenova. 2007. PMID: 18318178 Review. Russian.
Cited by
-
Heparin-like effect of a dual antiplatelet and anticoagulant (APAC) agent on red blood cell deformability and aggregation in an experimental model.J Thromb Thrombolysis. 2024 Dec;57(8):1329-1338. doi: 10.1007/s11239-024-03040-8. Epub 2024 Sep 4. J Thromb Thrombolysis. 2024. PMID: 39231863 Free PMC article.
References
-
- Bizjak D.A., John L., Matits L., Uhl A., Schulz S.V.W., Schellenberg J., Peifer J., Bloch W., Weiß M., Grüner B., et al. SARS-CoV-2 Altered Hemorheological and Hematological Parameters during One-Month Observation Period in Critically Ill COVID-19 Patients. Int. J. Mol. Sci. 2022;23:15332. doi: 10.3390/ijms232315332. - DOI - PMC - PubMed
-
- Ifrig E., Fibben K., Silvestri G., Maier C., Lam W. Fibrinogen-RBC Interactions Play a Key Role in COVID-19-Associated Endothelial Dysfunction. Chest. 2021;160:A1067. doi: 10.1016/j.chest.2021.07.987. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

