Neonatal Exposure to Lipopolysaccharide Promotes Neurogenesis of Subventricular Zone Progenitors in the Developing Neocortex of Ferrets
- PMID: 37834410
- PMCID: PMC10573966
- DOI: 10.3390/ijms241914962
Neonatal Exposure to Lipopolysaccharide Promotes Neurogenesis of Subventricular Zone Progenitors in the Developing Neocortex of Ferrets
Abstract
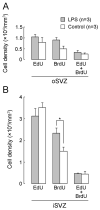
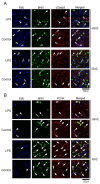
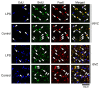
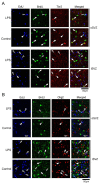
Lipopolysaccharide (LPS) is a natural agonist of toll-like receptor 4 that serves a role in innate immunity. The current study evaluated the LPS-mediated regulation of neurogenesis in the subventricular zone (SVZ) progenitors, that is, the basal radial glia and intermediate progenitors (IPs), in ferrets. Ferret pups were subcutaneously injected with LPS (500 μg/g of body weight) on postnatal days (PDs) 6 and 7. Furthermore, 5-ethynyl-2'-deoxyuridine (EdU) and 5-bromo-2'-deoxyuridine (BrdU) were administered on PDs 5 and 7, respectively, to label the post-proliferative and proliferating cells in the inner SVZ (iSVZ) and outer SVZ (oSVZ). A significantly higher density of BrdU single-labeled proliferating cells was observed in the iSVZ of LPS-exposed ferrets than in controls but not in post-proliferative EdU single-labeled and EdU/BrdU double-labeled self-renewing cells. BrdU single-labeled cells exhibited a lower proportion of Tbr2 immunostaining in LPS-exposed ferrets (22.2%) than in controls (42.6%) and a higher proportion of Ctip2 immunostaining in LPS-exposed ferrets (22.2%) than in controls (8.6%). The present findings revealed that LPS modified the neurogenesis of SVZ progenitors. Neonatal LPS exposure facilitates the proliferation of SVZ progenitors, followed by the differentiation of Tbr2-expressing IPs into Ctip2-expressing immature neurons.
Keywords: Ctip2; Tbr2; carnivores; intermediate progenitors; toll-like receptor.
Conflict of interest statement
There are no conflicts of interest.
Figures




Similar articles
-
Neurogenesis of Subventricular Zone Progenitors in the Premature Cortex of Ferrets Facilitated by Neonatal Valproic Acid Exposure.Int J Mol Sci. 2022 Apr 28;23(9):4882. doi: 10.3390/ijms23094882. Int J Mol Sci. 2022. PMID: 35563273 Free PMC article.
-
Follow-up study of subventricular zone progenitors with multiple rounds of cell division during sulcogyrogenesis in the ferret cerebral cortex.IBRO Rep. 2019 Aug 1;7:42-51. doi: 10.1016/j.ibror.2019.07.1720. eCollection 2019 Dec. IBRO Rep. 2019. PMID: 31453408 Free PMC article.
-
Tracking of Internal Granular Progenitors Responding to Valproic Acid in the Cerebellar Cortex of Infant Ferrets.Cells. 2024 Feb 7;13(4):308. doi: 10.3390/cells13040308. Cells. 2024. PMID: 38391920 Free PMC article.
-
The Subventricular Zone: A Key Player in Human Neocortical Development.Neuroscientist. 2018 Apr;24(2):156-170. doi: 10.1177/1073858417691009. Epub 2017 Feb 13. Neuroscientist. 2018. PMID: 29254416 Review.
-
Neural progenitors, neurogenesis and the evolution of the neocortex.Development. 2014 Jun;141(11):2182-94. doi: 10.1242/dev.090571. Development. 2014. PMID: 24866113 Review.
Cited by
-
Altered Cerebral Cortical Gyrification in Ferrets with Neonatal Exposure to the Bacterial Endotoxin, Lipopolysaccharide.eNeuro. 2025 Aug 7;12(8):ENEURO.0135-25.2025. doi: 10.1523/ENEURO.0135-25.2025. Print 2025 Aug. eNeuro. 2025. PMID: 40695594 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

