Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age
- PMID: 37834424
- PMCID: PMC10573797
- DOI: 10.3390/ijms241914977
Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age
Abstract
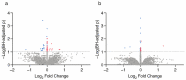
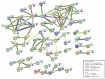
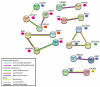
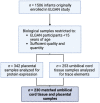
Prenatal exposure to toxic metals is associated with altered placental function and adverse infant and child health outcomes. Adverse outcomes include those that are observed at the time of birth, such as low birthweight, as well as those that arise later in life, such as neurological impairment. It is often the case that these adverse outcomes show sex-specific responses in relation to toxicant exposures. While the precise molecular mechanisms linking in utero toxic metal exposures with later-in-life health are unknown, placental inflammation is posited to play a critical role. Here, we sought to understand whether in utero metal exposure is associated with alterations in the expression of the placental proteome by identifying metal associated proteins (MAPs). Within the Extremely Low Gestational Age Newborns (ELGAN) cohort (n = 230), placental and umbilical cord tissue samples were collected at birth. Arsenic (As), cadmium (Cd), lead (Pb), selenium (Se), and manganese (Mn) concentrations were measured in umbilical cord tissue samples via ICP-MS/MS. Protein expression was examined in placental samples using an LC-MS/MS-based, global, untargeted proteomics analysis measuring more than 3400 proteins. MAPs were then evaluated for associations with pregnancy and neonatal outcomes, including placental weight and gestational age. We hypothesized that metal levels would be positively associated with the altered expression of inflammation/immune-associated pathways and that sex-specific patterns of metal-associated placental protein expression would be observed. Sex-specific analyses identified 89 unique MAPs expressed in female placentas and 41 unique MAPs expressed in male placentas. Notably, many of the female-associated MAPs are known to be involved in immune-related processes, while the male-associated MAPs are associated with intracellular transport and cell localization. Further, several MAPs were significantly associated with gestational age in males and females and placental weight in males. These data highlight the linkage between prenatal metal exposure and an altered placental proteome, with implications for altering the trajectory of fetal development.
Keywords: metals; placenta; preterm birth; proteomics; sexual dimorphism; umbilical cord tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Arsenic. 2018. [(accessed on 23 January 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic.
-
- Bulka C.M., Scannell B.M., Lombard M.A., Bartell S.M., Jones D.K., Bradley P.M., Vieira V.M., Silverman D.T., Focazio M., Toccalino P.M., et al. Arsenic in private well water and birth outcomes in the United States. Environ. Int. 2022;163:107176. doi: 10.1016/j.envint.2022.107176. - DOI - PMC - PubMed
-
- Eaves L.A., Keil A.P., Rager J.E., George A., Fry R.C. Analysis of the novel NCWELL database highlights two decades of co-occurrence of toxic metals in North Carolina private well water: Public health and environmental justice implications. Sci. Total Environ. 2022;812:151479. doi: 10.1016/j.scitotenv.2021.151479. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

