Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs
- PMID: 37834429
- PMCID: PMC10573759
- DOI: 10.3390/ijms241914983
Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs
Abstract
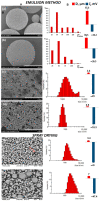
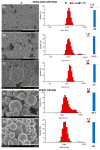
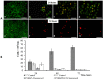
The development of controlled drug delivery systems, in the form of microparticles, is an important area of experimental pharmacology. The success of the design and the quality of the obtained microparticles are determined by the method of manufacture and the properties of the material used as a carrier. The goal is to obtain and characterize microparticles depending on their method of preparation, the chemical composition of the polymer and the load of the drugs. To obtain microparticles, four types of degradable PHAs, differing in their chemical compositions, degrees of crystallinity, molecular weights and temperature characteristics, were used (poly-3-hydroxybutyrate and copolymers 3-hydroxybutyric-co-3-hydroxyvaleric acid, 3-hydroxybutyric-co-4-hydroxybutyric acid, and 3-hydroxybutyric-co-3-hydroxyhexanoic acid). The characteristics of microparticles from PHAs were studied. Good-quality particles with an average particle diameter from 0.8 to 65.0 μm, having satisfactory ζ potential values (from -18 to -50 mV), were obtained. The drug loading content, encapsulation efficiency and in vitro release were characterized. Composite microparticles based on PHAs with additives of polyethylene glycol and polylactide-co-glycolide, and loaded with ceftriaxone and 5-fluorouracil, showed antibacterial and antitumor effects in E. coli and HeLa cultures. The results indicate the high potential of PHAs for the design of modern and efficient drug delivery systems.
Keywords: E. coli and HeLa cultures; biodegradable polyhydroxyalkanoates; drug delivery systems; drug efficiency; microparticles; properties.
Conflict of interest statement
The authors declare that they have no conflict of interest in the publication of this article. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Figures



Similar articles
-
Chitosan-modified ceftazidime loaded polyhydroxyalkanoates microparticles: preparation, characterization and antibacterial evaluation in vitro.ADMET DMPK. 2025 Mar 18;13(2):2645. doi: 10.5599/admet.2645. eCollection 2025. ADMET DMPK. 2025. PMID: 40314005 Free PMC article.
-
Microparticles prepared from biodegradable polyhydroxyalkanoates as matrix for encapsulation of cytostatic drug.J Mater Sci Mater Med. 2013 Aug;24(8):1905-15. doi: 10.1007/s10856-013-4941-2. Epub 2013 May 15. J Mater Sci Mater Med. 2013. PMID: 23674057
-
Preparation and Characterization of Doripenem-Loaded Microparticles for Pulmonary Delivery.J Aerosol Med Pulm Drug Deliv. 2018 Dec;31(6):347-357. doi: 10.1089/jamp.2017.1378. Epub 2018 Jun 7. J Aerosol Med Pulm Drug Deliv. 2018. PMID: 29877747
-
Biomedical applications of microbially engineered polyhydroxyalkanoates: an insight into recent advances, bottlenecks, and solutions.Appl Microbiol Biotechnol. 2019 Mar;103(5):2007-2032. doi: 10.1007/s00253-018-09604-y. Epub 2019 Jan 15. Appl Microbiol Biotechnol. 2019. PMID: 30645689 Review.
-
Current trends in polyhydroxyalkanoates (PHAs) biosynthesis: insights from the recombinant Escherichia coli.J Biotechnol. 2014 Jun 20;180:52-65. doi: 10.1016/j.jbiotec.2014.03.020. Epub 2014 Mar 31. J Biotechnol. 2014. PMID: 24698847 Review.
Cited by
-
Effect of surfactants and polymer composition on the characteristics of polyhydroxyalkanoate nanoparticles.ADMET DMPK. 2025 Jun 4;13(3):2723. doi: 10.5599/admet.2723. eCollection 2025. ADMET DMPK. 2025. PMID: 40585414 Free PMC article.
-
Chitosan-modified ceftazidime loaded polyhydroxyalkanoates microparticles: preparation, characterization and antibacterial evaluation in vitro.ADMET DMPK. 2025 Mar 18;13(2):2645. doi: 10.5599/admet.2645. eCollection 2025. ADMET DMPK. 2025. PMID: 40314005 Free PMC article.
-
Spray-dried cyclophosphamide-loaded polyhydroxyalkanoate microparticles: design and characterization.ADMET DMPK. 2024 Oct 9;12(6):925-942. doi: 10.5599/admet.2434. eCollection 2024. ADMET DMPK. 2024. PMID: 39713252 Free PMC article.
-
Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders.Plants (Basel). 2024 Jun 7;13(12):1584. doi: 10.3390/plants13121584. Plants (Basel). 2024. PMID: 38931016 Free PMC article. Review.
References
-
- Amass W., Amass A., Tighe B. A Review of Biodegradale Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym. Int. 1998;47:89–144. doi: 10.1002/(SICI)1097-0126(1998100)47:2<89::AID-PI86>3.0.CO;2-F. - DOI
-
- Poncelet D. Microencapsulation: Fundamentals, methods and applications. In: Blitz J., editor. Surface Chemistry in Biomedical and Environmental Science. Springer; Cham, The Netherlands: 2005. pp. 23–34.
-
- Panarin E.F., Lavrov N.A., Solovskii M.V., Shalnova L.I. Polymers—Carriers of biologically active substances. Publishing House of the COP “Profession”; St. Petersburg, Russia: 2014. p. 304.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

