Two Different Isocitrate Dehydrogenases from Pseudomonas aeruginosa: Enzymology and Coenzyme-Evolutionary Implications
- PMID: 37834433
- PMCID: PMC10574006
- DOI: 10.3390/ijms241914985
Two Different Isocitrate Dehydrogenases from Pseudomonas aeruginosa: Enzymology and Coenzyme-Evolutionary Implications
Abstract
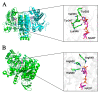
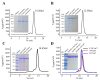
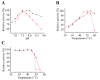
Pseudomonas aeruginosa PAO1, as an experimental model for Gram-negative bacteria, harbors two NADP+-dependent isocitrate dehydrogenases (NADP-IDHs) that were evolved from its ancient counterpart NAD-IDHs. For a better understanding of PaIDH1 and PaIDH2, we cloned the genes, overexpressed them in Escherichia coli and purified them to homogeneity. PaIDH1 displayed higher affinity to NADP+ and isocitrate, with lower Km values when compared to PaIDH2. Moreover, PaIDH1 possessed higher temperature tolerance (50 °C) and wider pH range tolerance (7.2-8.5) and could be phosphorylated. After treatment with the bifunctional PaIDH kinase/phosphatase (PaIDH K/P), PaIDH1 lost 80% of its enzymatic activity in one hour due to the phosphorylation of Ser115. Small-molecule compounds like glyoxylic acid and oxaloacetate can effectively inhibit the activity of PaIDHs. The mutant PaIDH1-D346I347A353K393 exhibited enhanced affinity for NAD+ while it lost activity towards NADP+, and the Km value (7770.67 μM) of the mutant PaIDH2-L589 I600 for NADP+ was higher than that observed for NAD+ (5824.33 μM), indicating a shift in coenzyme specificity from NADP+ to NAD+ for both PaIDHs. The experiments demonstrated that the mutation did not alter the oligomeric state of either protein. This study provides a foundation for the elucidation of the evolution and function of two NADP-IDHs in the pathogenic bacterium P. aeruginosa.
Keywords: Pseudomonas aeruginosa; coenzyme specificity; evolution; isocitrate dehydrogenase; phosphorylation.
Conflict of interest statement
The authors have no conflict of interest to declare that are relevant to the content of this article.
Figures



References
-
- Luckett J.C., Darch O., Watters C., Abuoun M., Wright V., Paredes-Osses E., Ward J., Goto H., Heeb S., Pommier S., et al. A novel virulence strategy for Pseudomonas aeruginosa mediated by an autotransporter with arginine-specific aminopeptidase activity. PLoS Pathog. 2012;8:e1002854. doi: 10.1371/journal.ppat.1002854. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32071270/National Natural Science Foundation of China
- 202003a06020009/Major Science and Technology Projects in Anhui Province
- 2022AH010012/Outstanding Innovative Research Team for Molecular Enzymology and Detection in Anhui Pro-vincial Universities
- 2208085MC48/Anhui Natural Science Foundation
- Auhui Provincial Engineering Research Centre for Molecular Detection and Diagnostics
LinkOut - more resources
Full Text Sources

