MicroRNA as Possible Mediators of the Synergistic Effect of Celecoxib and Glucosamine Sulfate in Human Osteoarthritic Chondrocyte Exposed to IL-1β
- PMID: 37834442
- PMCID: PMC10573984
- DOI: 10.3390/ijms241914994
MicroRNA as Possible Mediators of the Synergistic Effect of Celecoxib and Glucosamine Sulfate in Human Osteoarthritic Chondrocyte Exposed to IL-1β
Abstract
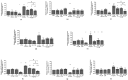
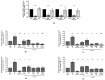
This study investigated the role of a pattern of microRNA (miRNA) as possible mediators of celecoxib and prescription-grade glucosamine sulfate (GS) effects in human osteoarthritis (OA) chondrocytes. Chondrocytes were treated with celecoxib (1.85 µM) and GS (9 µM), alone or in combination, for 24 h, with or without interleukin (IL)-1β (10 ng/mL). Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, apoptosis and reactive oxygen species (ROS) by cytometry, nitric oxide (NO) by Griess method. Gene levels of miRNA, antioxidant enzymes, nuclear factor erythroid (NRF)2, and B-cell lymphoma (BCL)2 expressions were analyzed by quantitative real time polymerase chain reaction (real time PCR). Protein expression of NRF2 and BCL2 was also detected at immunofluorescence and western blot. Celecoxib and GS, alone or in combination, significantly increased viability, reduced apoptosis, ROS and NO production and the gene expression of miR-34a, -146a, -181a, -210, in comparison to baseline and to IL-1β. The transfection with miRNA specific inhibitors significantly counteracted the IL-1β activity and potentiated the properties of celecoxib and GS on viability, apoptosis and oxidant system, through nuclear factor (NF)-κB regulation. The observed effects were enhanced when the drugs were tested in combination. Our data confirmed the synergistic anti-inflammatory and chondroprotective properties of celecoxib and GS, suggesting microRNA as possible mediators.
Keywords: NF-κB; celecoxib; chondrocytes; chondroprotection; glucosamine sulfate; inflammation; microRNA; osteoarthritis.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures







References
-
- Favero M., El-Hadi H., Belluzzi E., Granzotto M., Porzionato A., Sarasin G., Rambaldo A., Iacobellis C., Cigolotti A., Fontanella C.G., et al. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology. 2017;1:1784–1793. doi: 10.1093/rheumatology/kex287. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

