Frontotemporal Dementia P301L Mutation Potentiates but Is Not Sufficient to Cause the Formation of Cytotoxic Fibrils of Tau
- PMID: 37834443
- PMCID: PMC10573866
- DOI: 10.3390/ijms241914996
Frontotemporal Dementia P301L Mutation Potentiates but Is Not Sufficient to Cause the Formation of Cytotoxic Fibrils of Tau
Abstract
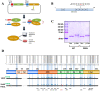
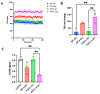
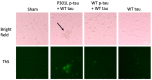
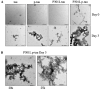
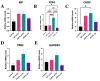
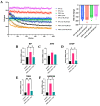
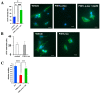
The P301L mutation in tau protein is a prevalent pathogenic mutation associated with neurodegenerative frontotemporal dementia, FTD. The mechanism by which P301L triggers or facilitates neurodegeneration at the molecular level remains unclear. In this work, we examined the effect of the P301L mutation on the biochemical and biological characteristics of pathologically relevant hyperphosphorylated tau. Hyperphosphorylated P301L tau forms cytotoxic aggregates more efficiently than hyperphosphorylated wildtype tau or unphosphorylated P301L tau in vitro. Mechanistic studies establish that hyperphosphorylated P301L tau exacerbates endoplasmic reticulum (ER) stress-associated gene upregulation in a neuroblastoma cell line when compared to wildtype hyperphosphorylated tau treatment. Furthermore, the microtubule cytoskeleton is severely disrupted following hyperphosphorylated P301L tau treatment. A hyperphosphorylated tau aggregation inhibitor, apomorphine, also inhibits the harmful effects caused by P301L hyperphosphorylated tau. In short, the P301L single mutation within the core repeat domain of tau renders the underlying hyperphosphorylated tau more potent in eliciting ER stress and cytoskeleton damage. However, the P301L mutation alone, without hyperphosphorylation, is not sufficient to cause these phenotypes. Understanding the conditions and mechanisms whereby selective mutations aggravate the pathogenic activities of tau can provide pivotal clues on novel strategies for drug development for frontotemporal dementia and other related neurodegenerative tauopathies, including Alzheimer's disease.
Keywords: Alzheimer’s disease; P301L; apomorphine; frontotemporal dementia; hyperphosphorylated tau; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Combining P301L and S320F tau variants produces a novel accelerated model of tauopathy.Hum Mol Genet. 2019 Oct 1;28(19):3255-3269. doi: 10.1093/hmg/ddz151. Hum Mol Genet. 2019. PMID: 31261380 Free PMC article.
-
Early depletion of CA1 neurons and late neurodegeneration in a mouse tauopathy model.Brain Res. 2017 Jun 15;1665:22-35. doi: 10.1016/j.brainres.2017.04.002. Epub 2017 Apr 11. Brain Res. 2017. PMID: 28411086
-
Genotype-specific differences between mouse CNS stem cell lines expressing frontotemporal dementia mutant or wild type human tau.PLoS One. 2012;7(6):e39328. doi: 10.1371/journal.pone.0039328. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723997 Free PMC article.
-
Tau and neurodegeneration.Cytoskeleton (Hoboken). 2024 Jan;81(1):95-102. doi: 10.1002/cm.21812. Epub 2023 Dec 10. Cytoskeleton (Hoboken). 2024. PMID: 38073060 Free PMC article. Review.
-
Tauopathies as clinicopathological entities.Parkinsonism Relat Disord. 2016 Jan;22 Suppl 1(0 1):S29-33. doi: 10.1016/j.parkreldis.2015.09.020. Epub 2015 Sep 8. Parkinsonism Relat Disord. 2016. PMID: 26382841 Free PMC article. Review.
Cited by
-
Top 100 most-cited articles on tau protein: a bibliometric analysis and evidence mapping.Front Neurosci. 2024 Jan 31;18:1345225. doi: 10.3389/fnins.2024.1345225. eCollection 2024. Front Neurosci. 2024. PMID: 38356652 Free PMC article.
-
Hyperphosphorylated tau-based Alzheimer's Disease drug discovery: Identification of inhibitors of tau aggregation and cytotoxicity.SLAS Discov. 2025 Jun;33:100235. doi: 10.1016/j.slasd.2025.100235. Epub 2025 May 3. SLAS Discov. 2025. PMID: 40319815 Free PMC article.
-
Animal models of Alzheimer's disease: Current strategies and new directions.Zool Res. 2024 Nov 18;45(6):1385-1407. doi: 10.24272/j.issn.2095-8137.2024.274. Zool Res. 2024. PMID: 39572020 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

