Insulin Receptor Isoforms and Insulin Growth Factor-like Receptors: Implications in Cell Signaling, Carcinogenesis, and Chemoresistance
- PMID: 37834454
- PMCID: PMC10573852
- DOI: 10.3390/ijms241915006
Insulin Receptor Isoforms and Insulin Growth Factor-like Receptors: Implications in Cell Signaling, Carcinogenesis, and Chemoresistance
Abstract
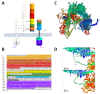
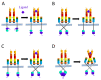
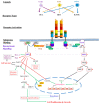
This comprehensive review thoroughly explores the intricate involvement of insulin receptor (IR) isoforms and insulin-like growth factor receptors (IGFRs) in the context of the insulin and insulin-like growth factor (IGF) signaling (IIS) pathway. This elaborate system encompasses ligands, receptors, and binding proteins, giving rise to a wide array of functions, including aspects such as carcinogenesis and chemoresistance. Detailed genetic analysis of IR and IGFR structures highlights their distinct isoforms, which arise from alternative splicing and exhibit diverse affinities for ligands. Notably, the overexpression of the IR-A isoform is linked to cancer stemness, tumor development, and resistance to targeted therapies. Similarly, elevated IGFR expression accelerates tumor progression and fosters chemoresistance. The review underscores the intricate interplay between IRs and IGFRs, contributing to resistance against anti-IGFR drugs. Consequently, the dual targeting of both receptors could present a more effective strategy for surmounting chemoresistance. To conclude, this review brings to light the pivotal roles played by IRs and IGFRs in cellular signaling, carcinogenesis, and therapy resistance. By precisely modulating these receptors and their complex signaling pathways, the potential emerges for developing enhanced anti-cancer interventions, ultimately leading to improved patient outcomes.
Keywords: IGF signal transduction; chemoresistance; insulin growth factor-like receptors; insulin receptor isoforms; insulin signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

