Multi-Omics Profiling of Human Endothelial Cells from the Coronary Artery and Internal Thoracic Artery Reveals Molecular but Not Functional Heterogeneity
- PMID: 37834480
- PMCID: PMC10573276
- DOI: 10.3390/ijms241915032
Multi-Omics Profiling of Human Endothelial Cells from the Coronary Artery and Internal Thoracic Artery Reveals Molecular but Not Functional Heterogeneity
Abstract
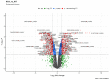
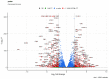
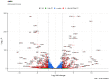
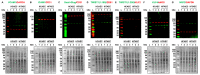
Major adverse cardiovascular events occurring upon coronary artery bypass graft surgery are typically accompanied by endothelial dysfunction. Total arterial revascularisation, which employs both left and right internal thoracic arteries instead of the saphenous vein to create a bypass, is associated with better mid- and long-term outcomes. We suggested that molecular profiles of human coronary artery endothelial cells (HCAECs) and human internal mammary artery endothelial cells (HITAECs) are coherent in terms of transcriptomic and proteomic signatures, which were then investigated by RNA sequencing and ultra-high performance liquid chromatography-mass spectrometry, respectively. Both HCAECs and HITAECs overexpressed molecules responsible for the synthesis of extracellular matrix (ECM) components, basement membrane assembly, cell-ECM adhesion, organisation of intercellular junctions, and secretion of extracellular vesicles. HCAECs were characterised by higher enrichment with molecular signatures of basement membrane construction, collagen biosynthesis and folding, and formation of intercellular junctions, whilst HITAECs were notable for augmented pro-inflammatory signaling, intensive synthesis of proteins and nitrogen compounds, and enhanced ribosome biogenesis. Despite HCAECs and HITAECs showing a certain degree of molecular heterogeneity, no specific markers at the protein level have been identified. Coherence of differentially expressed molecular categories in HCAECs and HITAECs suggests synergistic interactions between these ECs in a bypass surgery scenario.
Keywords: RNA sequencing; coronary artery; coronary artery bypass graft surgery; endothelial cells; endothelial heterogeneity; global gene expression; internal thoracic artery; multi-omics; proteomic profiling; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Proteomic Profiling of Endothelial Cell Secretomes After Exposure to Calciprotein Particles Reveals Downregulation of Basement Membrane Assembly and Increased Release of Soluble CD59.Int J Mol Sci. 2024 Oct 23;25(21):11382. doi: 10.3390/ijms252111382. Int J Mol Sci. 2024. PMID: 39518935 Free PMC article.
-
Proteomic Profiling of Endothelial Cells Exposed to Mitomycin C: Key Proteins and Pathways Underlying Genotoxic Stress-Induced Endothelial Dysfunction.Int J Mol Sci. 2024 Apr 5;25(7):4044. doi: 10.3390/ijms25074044. Int J Mol Sci. 2024. PMID: 38612854 Free PMC article.
-
Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions.Int J Mol Sci. 2020 Oct 28;21(21):8032. doi: 10.3390/ijms21218032. Int J Mol Sci. 2020. PMID: 33126651 Free PMC article.
-
What is the best graft to supplement the bilateral internal thoracic artery to the left coronary system? A meta-analysis.Eur J Cardiothorac Surg. 2019 Jul 1;56(1):21-29. doi: 10.1093/ejcts/ezy476. Eur J Cardiothorac Surg. 2019. PMID: 30649244
-
Reusing the patent internal mammary artery as a conduit in redo coronary artery bypass surgery.Interact Cardiovasc Thorac Surg. 2016 Mar;22(3):346-50. doi: 10.1093/icvts/ivv338. Epub 2015 Dec 15. Interact Cardiovasc Thorac Surg. 2016. PMID: 26669852 Free PMC article. Review.
Cited by
-
Quantitative analysis of the proteome and protein oxidative modifications in primary human coronary artery endothelial cells and associated extracellular matrix.Redox Biol. 2025 Apr;81:103524. doi: 10.1016/j.redox.2025.103524. Epub 2025 Feb 6. Redox Biol. 2025. PMID: 39954365 Free PMC article.
-
Calciprotein Particles Induce Cellular Compartment-Specific Proteome Alterations in Human Arterial Endothelial Cells.J Cardiovasc Dev Dis. 2023 Dec 22;11(1):5. doi: 10.3390/jcdd11010005. J Cardiovasc Dev Dis. 2023. PMID: 38248875 Free PMC article.
-
Proteomic Profiling of Endothelial Cell Secretomes After Exposure to Calciprotein Particles Reveals Downregulation of Basement Membrane Assembly and Increased Release of Soluble CD59.Int J Mol Sci. 2024 Oct 23;25(21):11382. doi: 10.3390/ijms252111382. Int J Mol Sci. 2024. PMID: 39518935 Free PMC article.
-
Proteomic Profiling of Endothelial Cells Exposed to Mitomycin C: Key Proteins and Pathways Underlying Genotoxic Stress-Induced Endothelial Dysfunction.Int J Mol Sci. 2024 Apr 5;25(7):4044. doi: 10.3390/ijms25074044. Int J Mol Sci. 2024. PMID: 38612854 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

