Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies
- PMID: 37834768
- PMCID: PMC10573998
- DOI: 10.3390/jcm12196124
Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies
Abstract
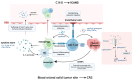
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the treatment of malignancies, especially hematological tumors, but toxicities have tempered its success. The main impediments to the development of CAR-T cell therapies are the following: cytokine release syndrome (CRS), immune-effector-cell-associated neurotoxicity syndrome (ICANS), tumor lysis syndrome (TLS), and on-target/off-tumor toxicity (OTOT). This review summarizes these side effects' underlying mechanisms and manifestations over time. It provides potential prevention and treatment according to the consensus grading, stressing the significance of establishing strategies that anticipate, reduce, and navigate the beginning of these side effects. It is essential to fully comprehend the mechanisms underlying these toxicities to create efficient treatment and preventive approaches.
Keywords: CAR-T cell; cytokine release syndrome; immune-effector-cell-associated neurotoxicity syndrome; management; mechanisms; strategies.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures



References
Publication types
Grants and funding
- 81802468/National Natural Science Foundation of China
- 2023YFS0088/Science and Technological Supports Project of Sichuan Province
- 2023NSFSC0535/Science and Technological Supports Project of Sichuan Province
- 2023YFS0095/Sichuan Science and Technology Program
- 2021M702346/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources

