Dialysis Patients Respond Adequately to Influenza Vaccination Irrespective of Dialysis Modality and Chronic Inflammation
- PMID: 37834849
- PMCID: PMC10573409
- DOI: 10.3390/jcm12196205
Dialysis Patients Respond Adequately to Influenza Vaccination Irrespective of Dialysis Modality and Chronic Inflammation
Abstract
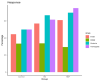
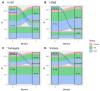
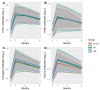
(1) Background: Chronic inflammation and suboptimal immune responses to vaccinations are considered to be aspects of immune dysregulation in patients that are undergoing dialysis. The present study aimed to evaluate immune responses in hemodialysis (HD) and online hemodiafiltration (OL-HDF) patients to a seasonal inactivated quadrivalent influenza vaccine (IQIV). (2) Methods: We enrolled 172 chronic dialysis patients (87 on HD and 85 on OL-HDF) and 18 control subjects without chronic kidney disease in a prospective, cross-sectional cohort study. Participants were vaccinated with a seasonal IQIV, and antibody titers using the hemagglutination inhibition (HI) assay were determined before vaccination (month 0) and 1, 3, and 6 months thereafter. Demographics and inflammatory markers (CRP, IL-6, IL-1β) were recorded at month 0. The primary endpoints were the rates of seroresponse (SR), defined as a four-fold increase in the HI titer, and seroprotection (SP), defined as HI titer ≥ 1/40 throughout the study period. Statistical analyses were conducted in R (version 3.6.3) statistical software. The differences between groups were analyzed using chi-square and t-test analyses for dichotomous and continuous variables, respectively. To identify independent determinants of SR and SP, generalized linear models were built with response or protection per virus strain as the dependent variable and group, age, sex, time (month 0, 1, 3, 6), diabetes, IL-6, dialysis vintage, HD access, and HDF volume as independent explanatory variables. (3) Results: SR and SP rates were similar between control subjects, and dialysis patients were not affected by dialysis modality. SP rates were high (> 70%) at the beginning of the study and practically reached 100% after vaccination in all study groups. These results applied to all four virus strains that were included in the IQIV. IL-6 levels significantly differed between study groups, with HD patients displaying the highest values, but this did not affect SP rates. (4) Conclusions: Dialysis patients respond to influenza immunization adequately and similarly to the general population. Thus, annual vaccination policies should be encouraged in dialysis units. OL-HDF reduces chronic inflammation; however, this has no impact on SR rates.
Keywords: chronic inflammation; dialysis; hemodiafiltration; immune response; influenza vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Buchy P., Buisson Y., Cintra O., Dwyer D.E., Nissen M., de Lejarazu R.O., Petersen E. COVID-19 pandemic: Lessons learned from more than a century of pandemics and current vaccine development for pandemic control. Int. J. Infect. Dis. 2021;112:300–317. doi: 10.1016/j.ijid.2021.09.045. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

