Transcatheter Aortic Valve Replacement in Degenerated Perceval Bioprosthesis: Clinical and Technical Aspects in 32 Cases
- PMID: 37834910
- PMCID: PMC10573422
- DOI: 10.3390/jcm12196265
Transcatheter Aortic Valve Replacement in Degenerated Perceval Bioprosthesis: Clinical and Technical Aspects in 32 Cases
Abstract
Background: Sutureless aortic bioprostheses are increasingly being used to provide shorter cross-clamp time and facilitate minimally invasive aortic valve replacement. As the use of sutureless valves has increased over the past decade, we begin to encounter their degeneration. We describe clinical outcomes and technical aspects in patients with degenerated sutureless Perceval (CorCym, Italy) aortic bioprosthesis treated with valve-in-valve transcatheter aortic valve replacement (VIV-TAVR).
Methods: Between March 2011 and March 2023, 1310 patients underwent aortic valve replacement (AVR) with Perceval bioprosthesis implantation. Severe bioprosthesis degeneration treated with VIV-TAVR occurred in 32 patients with a mean of 6.4 ± 1.9 years (range: 2-10 years) after first implantation. Mean EuroSCORE II was 9.5 ± 6.4% (range: 1.9-35.1%).
Results: Thirty of thirty-two (94%) VIV-TAVR were performed via transfemoral and two (6%) via transapical approach. Vascular complications occurred in two patients (6%), and mean hospital stay was 4.6 ± 2.4 days. At mean follow-up of 16.7 ± 15.2 months (range: 1-50 months), survival was 100%, and mean transvalvular pressure gradient was 18.7 ± 5.3 mmHg.
Conclusion: VIV-TAVR is a useful option for degenerated Perceval and appears safe and effective. This procedure is associated with good clinical results and excellent hemodynamic performance in our largest single-center experience.
Keywords: aortic bioprosthesis degeneration; aortic valve replacement; sutureless aortic valve; transcatheter aortic valve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
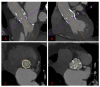
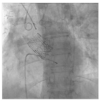
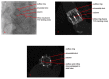

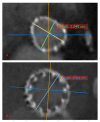
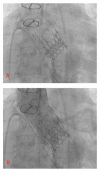
References
-
- Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., ErwinIII J.P., Guyton R.A., O’Gara P.T., Ruiz C.E., Skubas N.J., Sorajja P., et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:2438–2488. - PubMed
-
- Vahanian A., Alfieri O., Andreotti F., Antunes M.J., Barón-Esquivias G., Baumgartner H., Borger M.A., Carrel T.P., De Bonis M., Evangelista A., et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. J. Cardiothorac. Surg. 2012;42:S1–S44. - PubMed
-
- Brown J.M., O’Brien S.M., Wu C., Sikora J.A., Griffith B.P., Gammie J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. - DOI - PubMed
-
- Fischlein T., Meuris B., Hakim-Meibodi K., Misfeld M., Carrel T., Zembala M., Gaggianesi S., Madonna F., Laborde F., Asch F., et al. CAVALIER Trial Investigators. The sutureless aortic valve at 1 year: A large multicenter cohort study. J. Thorac. Cardiovasc. Surg. 2016;151:1617–1626.e4. doi: 10.1016/j.jtcvs.2015.12.064. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

