Transcatheter Closure of Perimembranous Ventricular Septal Defects Including Multifenestrated and Gerbode-Type Defects Using the Lifetech Konar Device
- PMID: 37835013
- PMCID: PMC10573930
- DOI: 10.3390/jcm12196370
Transcatheter Closure of Perimembranous Ventricular Septal Defects Including Multifenestrated and Gerbode-Type Defects Using the Lifetech Konar Device
Abstract
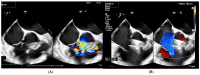
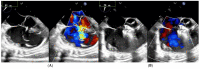
(1) Transcatheter closure of perimembranous ventricular septal defects (PmVSD) is becoming more attractive and effective with the development of new occluders. The aim of this study was to report a single-center experience in PmVSD closure using the Lifetech Konar-multifunctional occluder (MFO). (2) From March 2019 to October 2022, 43 consecutive patients were enrolled in the study. Among them, 13 had multifenestrated PmVSD including 5 Gerbode-type defects. (3) There were 23 males/20 females, and the median age was 17 years (range 2-68 years). Trivial aortic regurgitation was noticed in 19 patients. Implantation was successful in all patients under general anesthesia. A retrograde approach was used in 35 patients (81%). The retrograde approach was associated with a lower radiation dose (p = 0.042) and shorter fluoroscopy time (p = 0.002) compared to the antegrade approach. Full occlusion was observed immediately in 12 patients (28%) and in 33 patients (77%) at a median follow-up of 11 months. There were no complications such as embolization, complete atrioventricular block, device dislocation, new onset above grade I, or progression of tricuspid or aortic valve regurgitation. Seven of the thirteen patients with a multifenestrated defect had no residual shunt. The persistent shunts were all trivial intra-prosthetic leaks. (4) MFO is effective and safe for PmVSD closure including multifenestrated/Gerbode-type defects with no complication. However, a longer follow-up remains warranted to establish the safety of this technique.
Keywords: Konar-MFO device; congenital heart disease; pediatric cardiology; perimembranous VSD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Damsky Barbosa J., Alonso J., Ferrin L., Rivarola M., Lucini V., Biancolini J., Marques Vitorinio A., Ramirez R., Ackerman J., Martinez I., et al. Endovascular VSD closure with Lifetech Konar-multifunctional occluder-Novel device. Struct. Heart Dis. 2019;5:237–247.
-
- Haddad R.N., Daou L.S., Saliba Z.S. Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder: Midterm outcomes of the first middle-eastern experience. Catheter. Cardiovasc. Interv. 2020;96:E295–E302. doi: 10.1002/ccd.28678. - DOI - PubMed
-
- Kuswiyanto R.B., Gunawijaya E., Djer M.M., Noormanto Rahman M.A., Murni I.K., Sukardi R., Utamayasa A., Ardiansyah R., Nova R., Liliyanti S., et al. Transcatheter Closure of Perimembranous Ventricular Septal Defect Using the Lifetech Konar-Multi Functional Occluder: Early to midterm results of the Indonesian multicenter study. Glob. Heart. 2022;17:15. doi: 10.5334/gh.1106. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

