MET in Non-Small-Cell Lung Cancer (NSCLC): Cross 'a Long and Winding Road' Looking for a Target
- PMID: 37835473
- PMCID: PMC10571577
- DOI: 10.3390/cancers15194779
MET in Non-Small-Cell Lung Cancer (NSCLC): Cross 'a Long and Winding Road' Looking for a Target
Abstract
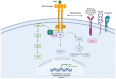
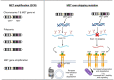
Non-Small-Cell Lung Cancer (NSCLC) can harbour different MET alterations, such as MET overexpression (MET OE), MET gene amplification (MET AMP), or MET gene mutations. Retrospective studies of surgical series of patients with MET-dysregulated NSCLC have shown worse clinical outcomes irrespective of the type of specific MET gene alteration. On the other hand, earlier attempts failed to identify the 'druggable' molecular gene driver until the discovery of MET exon 14 skipping mutations (METex14). METex14 are rare and amount to around 3% of all NSCLCs. Patients with METex14 NSCLC attain modest results when they are treated with immune checkpoint inhibitors (ICIs). New selective MET inhibitors (MET-Is) showed a long-lasting clinical benefit in patients with METex14 NSCLC and modest activity in patients with MET AMP NSCLC. Ongoing clinical trials are investigating new small molecule tyrosine kinase inhibitors, bispecific antibodies, or antibodies drug conjugate (ADCs). This review focuses on the prognostic role of MET, the summary of pivotal clinical trials of selective MET-Is with a focus on resistance mechanisms. The last section is addressed to future developments and challenges.
Keywords: MET; MET amplification; MET exon 14 skipping mutations; MET inhibitors; MET overexpression; NSCLC; immunotherapy; prognosis; resistance mechanism.
Conflict of interest statement
G.S. served as an advisor for Takeda outside of the submitted work. A.P. served as a consultant or advisor for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche/Genentech and received speaker fees from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Jansenn, Eli Lilly, Merck Sharp & Dohme, eCancer, and Medscape—all outside of the submitted work. F.d.M. has received advisory fees from Roche, Bristol-Myers Squibb, and AstraZeneca and consulting fees from Merck Sharp & Dohme—all outside of the submitted work. Other authors do not have potential conflicts of interest to report.
Figures

References
-
- Bussolino F., Di Renzo M.F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

