Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis
- PMID: 37835620
- PMCID: PMC10571529
- DOI: 10.3390/ani13193014
Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis
Abstract
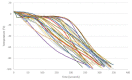
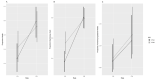
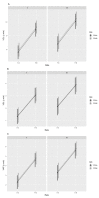
The cryopreservation and storage of gametes (biobanking) can provide a long-term, low-cost option for the preservation of population genetic diversity and is particularly impactful when applied to manage selective breeding within conservation breeding programs (CBPs). This study aimed to develop a sperm cryopreservation protocol for the critically endangered Booroolong frog (Litoria booroolongensis) to capture founder genetics within the recently established (est. 2019) CBP for this species. Hormone-induced sperm release was achieved using established protocols, and spermic urine samples were collected over a 6-h period. Pooled spermic urine samples (n = 3 males) were divided equally between two cryoprotectant (CPA) treatments and diluted by 1:5 (sperm:CPA) with either 15% (v/v) dimethyl sulfoxide + 1% (w/v) sucrose in simplified amphibian Ringer's (SAR; CPAA) or 10% (v/v) dimethylformamide + 10% (w/v) trehalose dihydrate in SAR (CPAB). The samples were cryopreserved in 0.25 mL straws using either a programmable freezer (FrA) or an adapted dry shipper method (FrB). The thawed samples were activated via dilution in water and assessed for viability and motility using both manual assessment and computer-assisted sperm analysis (CASA; 0 h, 0.5 h post-thaw). Upon activation, the survival and recovery of motility (total motility, forward progression and velocity) of cryopreserved sperm suspensions were higher for sperm preserved using FrB than FrA, regardless of CPA composition. This work supports our long-term goal to pioneer the integration of biobanked cryopreserved sperm with population genetic management to maximize restoration program outcomes for Australian amphibian species.
Keywords: ART; CASA; GRB; amphibian; biobanking; breeding program; conservation; reproductive technologies; spermiation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- International Union for Conservation of Nature Species Survival Commission, Amphibian Specialist Group . Amphibian Conservation Action Plan. IUCN; Gland, Switzerland: Cambridge, UK: 2007. p. 64.
-
- International Union for Conservation of Nature Species Survival Commission, Amphibian Specialist Group . The Amphibian Conservation Action Plan (ACAP): A Status Review and Roadmap for Global Amphibian Conservation. Volume Preprint The World Conservation Union (IUCN); Gland, Switzerland: 2022.
-
- Howell L.G., Mawson P.R., Frankham R., Rodger J.C., Upton R.M.O., Witt R.R., Calatayud N.E., Clulow S., Clulow J. Integrating biobanking could produce significant cost benefits and minimise inbreeding for Australian amphibian captive breeding programs. Reprod. Fertil. Dev. 2021;33:573–587. doi: 10.1071/RD21058. - DOI - PubMed
-
- Della Togna G., Howell L.G., Clulow J., Langhorne C.J., Marcec-Greaves R., Calatayud N.E. Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives. Theriogenology. 2020;150:412–431. doi: 10.1016/j.theriogenology.2020.02.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

