Does Vaccine-Induced Maternally-Derived Immunity Protect Swine Offspring against Influenza a Viruses? A Systematic Review and Meta-Analysis of Challenge Trials from 1990 to May 2021
- PMID: 37835692
- PMCID: PMC10571953
- DOI: 10.3390/ani13193085
Does Vaccine-Induced Maternally-Derived Immunity Protect Swine Offspring against Influenza a Viruses? A Systematic Review and Meta-Analysis of Challenge Trials from 1990 to May 2021
Abstract
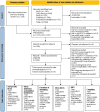
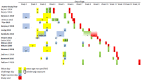
It is unclear if piglets benefit from vaccination of sows against influenza. For the first time, methods of evidence-based medicine were applied to answer the question: "Does vaccine-induced maternally-derived immunity (MDI) protect swine offspring against influenza A viruses?". Challenge trials were reviewed that were published from 1990 to April 2021 and measured at least one of six outcomes in MDI-positive versus MDI-negative offspring (hemagglutination inhibition (HI) titers, virus titers, time to begin and time to stop shedding, risk of infection, average daily gain (ADG), and coughing) (n = 15). Screening and extraction of study characteristics was conducted in duplicate by two reviewers, with data extraction and assessment for risk of bias performed by one. Homology was defined by the antigenic match of vaccine and challenge virus hemagglutinin epitopes. Results: Homologous, but not heterologous MDI, reduced virus titers in piglets. There was no difference, calculated as relative risks (RR), in infection incidence risk over the entire study period; however, infection hazard (instantaneous risk) was decreased in pigs with MDI (log HR = -0.64, 95% CI: -1.13, -0.15). Overall, pigs with MDI took about a ½ day longer to begin shedding virus post-challenge (MD = 0.51, 95% CI: 0.03, 0.99) but the hazard of infected pigs ceasing to shed was not different (log HR = 0.32, 95% CI: -0.29, 0.93). HI titers were synthesized qualitatively and although data on ADG and coughing was extracted, details were insufficient for conducting meta-analyses. Conclusion: Homology of vaccine strains with challenge viruses is an important consideration when assessing vaccine effectiveness. Herd viral dynamics are complex and may include concurrent or sequential exposures in the field. The practical significance of reduced weaned pig virus titers is, therefore, not known and evidence from challenge trials is insufficient to make inferences on the effects of MDI on incidence risk, time to begin or to cease shedding virus, coughing, and ADG. The applicability of evidence from single-strain challenge trials to field practices is limited. Despite the synthesis of six outcomes, challenge trial evidence does not support or refute vaccination of sows against influenza to protect piglets. Additional research is needed; controlled trials with multi-strain concurrent or sequential heterologous challenges have not been conducted, and sequential homologous exposure trials were rare. Consensus is also warranted on (1) the selection of core outcomes, (2) the sizing of trial populations to be reflective of field populations, (3) the reporting of antigenic characterization of vaccines, challenge viruses, and sow exposure history, and (4) on the collection of non-aggregated individual pig data.
Keywords: influenza A viruses of swine; influenza vaccines; maternally derived immunity; meta-analysis; systematic review.
Conflict of interest statement
The authors have no competing interests.
Figures








References
-
- Quesnel H., Farmer C., Devillers N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012;146:105–114. doi: 10.1016/j.livsci.2012.03.010. - DOI
-
- USDA National Animal Health Monitoring System (NAHMS) Swine 2012 Study. Part II: Reference of Swine Health and Health Management in the United States. USDA National Animal Health Monitoring System; Fort Collins, CO, USA: 2012. [(accessed on 1 June 2020)]. Available online: https://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2012....
-
- Moraes D.C.A.L., Vincent Baker A., Wang X., Zhu Z., Berg E., Trevisan G., Zhang J., Jayaraman S., Linhares D.C.L., Guager P.C., et al. Veterinarian perceptions and practices in prevention and control of influenza virus in the Midwest United States swine farms. Front. Vet. Sci. 2023;10:68. doi: 10.3389/fvets.2023.1089132. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

