Thermal Degradation Studies of Poly(2-ethyl hexyl acrylate) in the Presence of Nematic Liquid Crystals
- PMID: 37835983
- PMCID: PMC10575342
- DOI: 10.3390/polym15193934
Thermal Degradation Studies of Poly(2-ethyl hexyl acrylate) in the Presence of Nematic Liquid Crystals
Abstract
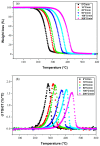
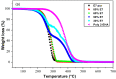
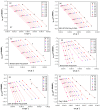
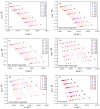
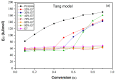
The thermal degradation behavior of Poly(2-ethyl hexyl hcrylate) (Poly(2-EHA)), blended with a commercially available nematic liquid crystal (LC) mixture, was investigated by thermal gravimetric analysis (TGA). Different heating rates, ranging from 5 to 200 °C/min, were applied under an inert atmosphere. Based on the TGA results, activation energies (Eα) at different conversion rates (α) were determined using three integral isoconversion methods: Flynn-Wall-Ozawa (FWO), Tang, and Kissinger-Akahira-Sunose (KAS). It can be noticed that the global evolution of these activation energies was the same for the three models. The coefficient of determination R2 presented values generally higher than 0.97. Using these models, the Eα value for the LC remains constant at 64 kJ/mol for all conversions rates. For the polymer Poly(2-EHA), applying the Tang and FWO models, the activation energy presents a variation ranging from 80 kJ/mol, for conversion α = 0.1, to 170 kJ/mol, for α = 0.9. For the third model (KAS), this energy varies between 80 and 220 kJ/mol in the same range of α.
Keywords: liquid crystal; non-isothermal method; polymer; thermal stability; thermogravimetric analysis.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Zhao C., Hu Y., Xu J., Yu M., Zou C., Wang Q., Gao Y., Yang H. Research on the Morphology, Electro-Optical Properties and Mechanical Properties of Electrochromic Polymer-Dispersed Liquid Crystalline Films Doped with Anthraquinone Dyes. Crystals. 2023;13:735. doi: 10.3390/cryst13050735. - DOI
-
- Lin H., Zhang S., Saeed M.H., Zhou L., Gao H., Huang J., Zhang L., Yang H., Xiao J., Gao Y. Effects of the methacrylate monomers with different end groups on the morphologies, electro-optical and mechanical properties of polymer dispersed liquid crystals composite films. Liq. Cryst. 2021;48:722–734. doi: 10.1080/02678292.2020.1815091. - DOI
-
- Hemaida A., Ghosh A., Sundaram S., Mallick T.K. Evaluation of thermal performance for a smart switchable adaptive polymer dispersed liquid crystal (PDLC) glazing. Sol. Energy. 2020;195:185–193. doi: 10.1016/j.solener.2019.11.024. - DOI
LinkOut - more resources
Full Text Sources

