Assessing Spectral Analysis of Phytoconstituents and Their In Silico Interactions with Target Proteins in Plant Seed Extracts
- PMID: 37836092
- PMCID: PMC10574034
- DOI: 10.3390/plants12193352
Assessing Spectral Analysis of Phytoconstituents and Their In Silico Interactions with Target Proteins in Plant Seed Extracts
Abstract
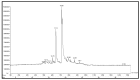
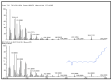
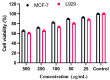
The pharmacological and preventive attributes of extracts from vegetable seeds have garnered widespread recognition within the scientific community. This study systematically assessed the in vitro antibacterial, antioxidant, and anti-breast cancer properties of phytochemicals present in various solvent-based vegetable seed extracts. We also conducted molecular docking simulations to ascertain their interactions with specific target proteins. Besides, nine distinct chemical constituents were identified using gas chromatography-mass spectrometry (GCMS). Remarkably, the ethyl acetate extract exhibited robust inhibitory effects against Gram-positive and Gram-negative bacterial strains. Furthermore, its capacity for 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging was found to be noteworthy, with an IC50 value of 550.82 ± 1.7 µg/mL, representing a scavenging efficiency of 64.1 ± 2.8%. Additionally, the ethyl acetate extract demonstrated significant hydrogen peroxide (H2O2) scavenging activity, with a maximal scavenging rate of 44.1 ± 1.70% (IC50) at a concentration of 761.17 ± 1.8 µg/mL. Intriguingly, in vitro cytotoxicity assays against human breast cancer (MCF-7) cells revealed varying levels of cell viability at different extract concentrations, suggesting potential anticancer properties. Importantly, these ethyl acetate extracts did not display toxicity to L929 cells across the concentration range tested. Subsequently, we conducted in-silico molecular docking experiments utilizing Discovery Studio 4.0 against the c-Met kinase protein (hepatocyte growth factor; PDB ID: 1N0W). Among the various compounds assessed, 3,4-Dihydroxy-1,6-bis-(3-methoxy-phenyl)-hexa-2,4-diene-1,6-dione exhibited a notable binding energy of -9.1 kcal/mol, warranting further investigation into its potential anticancer properties, clinical applications, and broader pharmacological characteristics.
Keywords: GC-MS; anti-breast cancer; antibacterial; antioxidant; in-silico molecular docking; vegetable seed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Stahl G.K., Angwin D.N., Very P., Gomes E., Weber Y., Tarba S.Y., Noorderhaven N., Benyamini H., Bouckenooghe D., Chreim S., et al. Sociocultural Integration in Mergers and Acquisitions: Unresolved Paradoxes and Directions for Future Research. Thunderbird Int. Bus. Rev. 2013;55:333–356. doi: 10.1002/tie.21549. - DOI
-
- Enzinger P.C., Mayer R.J. Esophageal Cancer. N. Engl. J. Med. 2004;350:1363–1364. doi: 10.1056/NEJMra035010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

