Hepatoprotective Effects of Four Brazilian Savanna Species on Acetaminophen-Induced Hepatotoxicity in HepG2 Cells
- PMID: 37836133
- PMCID: PMC10574628
- DOI: 10.3390/plants12193393
Hepatoprotective Effects of Four Brazilian Savanna Species on Acetaminophen-Induced Hepatotoxicity in HepG2 Cells
Abstract
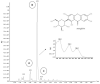
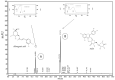
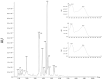
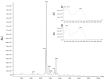
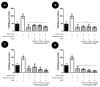
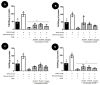
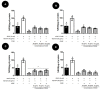
We investigated four Cerrado plant species, i.e., Cheiloclinium cognatum (Miers) A.C.Sm, Guazuma ulmifolia Lam., Hancornia speciosa Gomes, and Hymenaea stigonocarpa Mart. ex Hayne, against acetaminophen toxicity using an in vitro assay with HepG2 cells. The activity against acetaminophen toxicity was evaluated using different protocols, i.e., pre-treatment, co-treatment, and post-treatment of the cells with acetaminophen and the plant extracts. HepG2 cell viability after treatment with acetaminophen was 39.61 ± 5.59% of viable cells. In the pre-treatment protocol, the extracts could perform protection with viability ranging from 50.02 ± 15.24% to 78.75 ± 5.61%, approaching the positive control silymarin with 75.83 ± 5.52%. In the post-treatment protocol, all extracts and silymarin failed to reverse the acetaminophen damage. In the co-treatment protocol, the extracts showed protection ranging from 50.92 ± 11.14% to 68.50 ± 9.75%, and silymarin showed 77.87 ± 4.26%, demonstrating that the aqueous extracts of the species also do not increase the toxic effect of acetaminophen. This protection observed in cell viability was accompanied by a decrease in ROS. The extracts' hepatoprotection can be related to antioxidant compounds, such as rutin and mangiferin, identified using HPLC-DAD and UPLC-MS/MS. The extracts were shown to protect HepG2 cells against future APAP toxicity and may be candidates for supplements that could be used to prevent liver damage. In the concomitant treatment using the extracts with APAP, it was demonstrated that the extracts do not present a synergistic toxicity effect, with no occurrence of potentiation of toxicity. The extracts showed considerable cytoprotective effects and important antioxidant characteristics.
Keywords: acetaminophen; hepatoprotective activity; hepatotoxicity activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Levy B. Fisiologia. 6th ed. Elsevier; Rio de Janeiro, Brazil: 2009.
-
- Carvalho H.F. Células: Uma Abordagem Multidisciplinar. Manole; Barueri, Brazil: 2005. p. 450.
-
- Brasil . Cadernos de Atenção Básica—Práticas Integrativas e Complementares: Plantas Medicinais e Fitoterapia na Atenção Básica. 1st ed. Volume 31. Ministério da Saúde; Brasília, Brazil: 2012. p. 156.
LinkOut - more resources
Full Text Sources

