Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development
- PMID: 37836385
- PMCID: PMC10574741
- DOI: 10.3390/nu15194101
Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development
Abstract
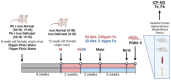
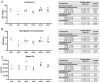
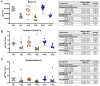
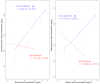
Maternal iron deficiency (ID) and environmental lead (Pb) exposure are co-occurring insults that both affect the neurodevelopment of offspring. Few studies have investigated how ID affects brain-region-specific Pb accumulations using human-relevant Pb concentrations. Furthermore, how these Pb exposures impact blood and brain Fe levels remains unclear. Importantly, we also wanted to determine whether the use of blood Pb levels as a surrogate for the brain Pb burden is affected by underlying iron status. We exposed virgin Swiss Webster female mice to one of six conditions differing by iron diet and Pb water concentration (0 ppm, 19 ppm, or 50 ppm lead acetate) and used Inductively Coupled Plasma Mass Spectrometry to measure the maternal and offspring circulating, stored, and brain Pb levels. We found that maternal ID rendered the offspring iron-deficient anemic and led to a region-specific depletion of brain Fe that was exacerbated by Pb in a dose-specific manner. The postnatal iron deficiency anemia also exacerbated cortical and hippocampal Pb accumulation. Interestingly, BPb levels only correlated with the brain Pb burden in ID pups but not in IN offspring. We conclude that ID significantly increases the brain Pb burden and that BPb levels alone are insufficient as a clinical surrogate to make extrapolations on the brain Pb burden.
Keywords: Pb burden; Swiss Webster; anemia; metals; micronutrients; neurodevelopment; pregnancy; risk assessment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
The effect of lead (Pb) exposure and iron (Fe) deficiency on intestinal lactobacilli, E. coli and yeast: A study in experimental rats.J Occup Health. 2018 Nov 27;60(6):475-484. doi: 10.1539/joh.2017-0267-OA. Epub 2018 Sep 11. J Occup Health. 2018. PMID: 30210097 Free PMC article.
-
Early-life iron deficiency persistently disrupts affective behaviour in mice.Ann Med. 2023 Dec;55(1):1265-1277. doi: 10.1080/07853890.2023.2191003. Ann Med. 2023. PMID: 37096819 Free PMC article.
-
Maternal iron deficiency alters essential fatty acid and eicosanoid metabolism and increases locomotion in adult guinea pig offspring.J Nutr. 2009 Sep;139(9):1653-9. doi: 10.3945/jn.109.106013. Epub 2009 Jul 29. J Nutr. 2009. PMID: 19640965
-
Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children.Environ Res. 2019 Oct;177:108641. doi: 10.1016/j.envres.2019.108641. Epub 2019 Aug 9. Environ Res. 2019. PMID: 31421445 Review.
-
Perinatal iron deficiency as an early risk factor for schizophrenia.Nutr Neurosci. 2022 Oct;25(10):2218-2227. doi: 10.1080/1028415X.2021.1943996. Epub 2021 Jun 24. Nutr Neurosci. 2022. PMID: 34165398 Free PMC article. Review.
Cited by
-
Cumulative risk assessment as the pathway to public health protection for behavioral neurotoxicity.Neurotoxicology. 2025 May;108:400-411. doi: 10.1016/j.neuro.2025.04.015. Epub 2025 May 10. Neurotoxicology. 2025. PMID: 40349850 Review.
References
-
- WHO . The Global Prevalence of Anaemia in 2011. WHO; Geneva, Switzerland: 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

