Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy
- PMID: 37836411
- PMCID: PMC10574198
- DOI: 10.3390/nu15194127
Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy
Abstract
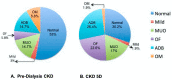
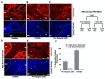
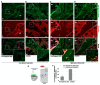
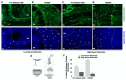
The pathophysiology of chronic kidney disease-mineral and bone disorder (CKD-MBD) is not well understood. Specific factors secreted by osteocytes are elevated in the serum of adults and pediatric patients with CKD-MBD, including FGF-23 and sclerostin, a known inhibitor of the Wnt signaling pathway. The molecular mechanisms that promote bone disease during the progression of CKD are incompletely understood. In this study, we performed a cross-sectional analysis of 87 pediatric patients with pre-dialysis CKD and post-dialysis (CKD 5D). We assessed the associations between serum and bone sclerostin levels and biomarkers of bone turnover and bone histomorphometry. We report that serum sclerostin levels were elevated in both early and late CKD. Higher circulating and bone sclerostin levels were associated with histomorphometric parameters of bone turnover and mineralization. Immunofluorescence analyses of bone biopsies evaluated osteocyte staining of antibodies towards the canonical Wnt target, β-catenin, in the phosphorylated (inhibited) or unphosphorylated (active) forms. Bone sclerostin was found to be colocalized with phosphorylated β-catenin, which suggests that Wnt signaling was inhibited. In patients with low serum sclerostin levels, increased unphosphorylated "active" β-catenin staining was observed in osteocytes. These data provide new mechanistic insight into the pathogenesis of CKD-MBD and suggest that sclerostin may offer a potential biomarker or therapeutic target in pediatric renal osteodystrophy.
Keywords: CKD-MBD; Wnt signaling; bone biopsy; children; immunofluorescence; immunohistochemistry; sclerostin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy.J Bone Miner Res. 2012 Aug;27(8):1757-72. doi: 10.1002/jbmr.1630. J Bone Miner Res. 2012. PMID: 22492547
-
The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease.Kidney Int. 2017 Jun;91(6):1436-1446. doi: 10.1016/j.kint.2016.12.029. Epub 2017 Mar 18. Kidney Int. 2017. PMID: 28318623
-
Sclerostin, Osteocytes, and Chronic Kidney Disease - Mineral Bone Disorder.Semin Dial. 2015 Nov-Dec;28(6):578-86. doi: 10.1111/sdi.12415. Epub 2015 Aug 19. Semin Dial. 2015. PMID: 26288182 Review.
-
Exercise Alleviates Osteoporosis in Rats with Mild Chronic Kidney Disease by Decreasing Sclerostin Production.Int J Mol Sci. 2019 Apr 25;20(8):2044. doi: 10.3390/ijms20082044. Int J Mol Sci. 2019. PMID: 31027235 Free PMC article.
-
From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD.Pediatr Nephrol. 2016 Feb;31(2):195-206. doi: 10.1007/s00467-015-3069-7. Epub 2015 Mar 4. Pediatr Nephrol. 2016. PMID: 25735207 Review.
Cited by
-
Increased Serum Sclerostin Level Is a Risk Factor for Peripheral Artery Disease in Patients with Hypertension.Medicina (Kaunas). 2025 Jul 1;61(7):1204. doi: 10.3390/medicina61071204. Medicina (Kaunas). 2025. PMID: 40731833 Free PMC article.
-
Evaluation of Bone Biomarkers in Renal Osteodystrophy.Life (Basel). 2024 Nov 25;14(12):1540. doi: 10.3390/life14121540. Life (Basel). 2024. PMID: 39768249 Free PMC article.
References
-
- Denburg M.R., Kumar J., Jemielita T., Brooks E.R., Skversky A., Portale A.A., Salusky I.B., Warady B.A., Furth S.L., Leonard M.B. Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. J. Am. Soc. Nephrol. 2016;27:543–550. doi: 10.1681/ASN.2015020152. - DOI - PMC - PubMed
-
- Smout D., Jørgensen H.S., Cavalier E., Evenepoel P. Clinical utility of bone turnover markers in patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2022;31:332–338. - PubMed
-
- Wagner J., Jhaveri K.D., Rosen L., Sunday S., Mathew A.T., Fishbane S. Increased bone fractures among elderly United States hemodialysis patients. Nephrol. Dial. Transplant. 2014;29:146–151. - PubMed

