Short-Range Charge Transfer in DNA Base Triplets: Real-Time Tracking of Coherent Fluctuation Electron Transfer
- PMID: 37836645
- PMCID: PMC10574627
- DOI: 10.3390/molecules28196802
Short-Range Charge Transfer in DNA Base Triplets: Real-Time Tracking of Coherent Fluctuation Electron Transfer
Abstract
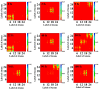
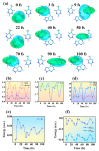
The short-range charge transfer of DNA base triplets has wide application prospects in bioelectronic devices for identifying DNA bases and clinical diagnostics, and the key to its development is to understand the mechanisms of short-range electron dynamics. However, tracing how electrons are transferred during the short-range charge transfer of DNA base triplets remains a great challenge. Here, by means of ab initio molecular dynamics and Ehrenfest dynamics, the nuclear-electron interaction in the thymine-adenine-thymine (TAT) charge transfer process is successfully simulated. The results show that the electron transfer of TAT has an oscillating phenomenon with a period of 10 fs. The charge density difference proves that the charge transfer proportion is as high as 59.817% at 50 fs. The peak position of the hydrogen bond fluctuates regularly between -0.040 and -0.056. The time-dependent Marcus-Levich-Jortner theory proves that the vibrational coupling between nucleus and electron induces coherent electron transfer in TAT. This work provides a real-time demonstration of the short-range coherent electron transfer of DNA base triplets and establishes a theoretical basis for the design and development of novel biological probe molecules.
Keywords: DNA base triplet; Ehrenfest dynamics; coherent electron transfer; nuclear-electron vibronic coupling; periodic oscillation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

