Determination of the Ionic Association Constants of Na+ with CO32- and HCO3- Ions, in NaCl-NaHCO3-H2O Ternary Systems, at 25 °C
- PMID: 37836656
- PMCID: PMC10574043
- DOI: 10.3390/molecules28196813
Determination of the Ionic Association Constants of Na+ with CO32- and HCO3- Ions, in NaCl-NaHCO3-H2O Ternary Systems, at 25 °C
Abstract
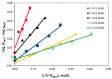
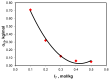
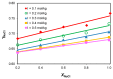
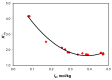
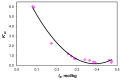
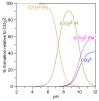
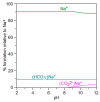
The ionic association constants of sodium with carbonate ion (K1C') and acidic carbonate ions (K2C') were measured in NaCl-NaHCO3-H2O ternary systems to determine the distribution of sodium among the chemical species present in the growth medium of Chlorella homosphaera 424 algae. The mean activity coefficients of sodium chloride (in pure sodium chloride and in a mixture of electrolytes) were determined experimentally using two electrochemical cells, namely Ag, AgCl| KCl (3 M)|| NH4NO3 (1 M)| NaCl (mNaCl)| Na+-ISE and Ag, AgCl|KCl (3 M)|| NH4NO3 (1 M)| NaCl (mNaCl)| Cl--ISE. The studies carried out show that the values of the association constants of K1C' and K2C' do not depend on the composition of the medium, but only on the effective ionic strength. The experimentally obtained γNaCl0 values in the binary system are comparable to the mean activity coefficients values for NaCl, calculated using data from the literature, with -0.9 to 0.1% relative standard deviation. The obtained results show that the experimentally determined mean activity coefficient in the ternary system, γNaCl, is smaller than γNaCl0 in the binary system over the entire field of ionic strengths studied. The ternary system NaCl-NaHCO3-H2O obeys Harned's rule.
Keywords: activity coefficients; algae growth media; association constant; effective ionic strength; ternary system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bilanovic D., Andargatchew A., Kroeger T., Shelef G. Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations—Response surface methodology analysis. Energy Convers. Manag. 2009;50:262–267. doi: 10.1016/j.enconman.2008.09.024. - DOI
-
- Houghton R. Why are estimates of the terrestrial carbon balance so different? Glob. Chang. Biol. 2003;9:500–509. doi: 10.1046/j.1365-2486.2003.00620.x. - DOI
-
- Neeraj, Yadav S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Technol. 2020;3:494–500. doi: 10.1016/j.mset.2020.03.005. - DOI
LinkOut - more resources
Full Text Sources

