Formation of 5-Aminomethyl-2,3-dihydropyridine-4(1 H)-ones from 4-Amino-tetrahydropyridinylidene Salts
- PMID: 37836712
- PMCID: PMC10574582
- DOI: 10.3390/molecules28196869
Formation of 5-Aminomethyl-2,3-dihydropyridine-4(1 H)-ones from 4-Amino-tetrahydropyridinylidene Salts
Abstract
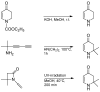
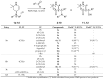
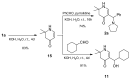
Various 4-aminotetrahydropyridinylidene salts were treated with aldehydes in an alkaline medium. Their conversion to 5-substituted β-hydroxyketones in a one-step reaction succeeded only with an aliphatic aldehyde. Instead, aromatic aldehydes gave 5-substituted β-aminoketones or a single δ-diketone. The new compounds were characterized using spectroscopic methods and a single crystal structure analysis. Some of them showed anticancer and antibacterial properties.
Keywords: antibacterial; anticancer activity; dihydropyridin-4(1H)-ones; tetrahydropyridinylidene salts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Seebacher W., Faist J., Belaj F., Saf R., Kaiser M., Brun R., Weis R. Synthesis of new tetrahydropyridinylidene ammonium salts and their antiprotozoal potency. Monatsh.Chem.. 2015;146:1299–1308. doi: 10.1007/s00706-015-1503-y. - DOI
-
- Mohsin N.-U., Seebacher W., Faist J., Hochegger P., Kaiser M., Mäser P., Belaj F., Saf R., Kretschmer N., Alajlani M., et al. Synthesis of new 1-benzyl tetrahydropyridinylidene ammonium salts and their antimicrobial and anticellular activities. Eur. J. Med. Chem. 2018;143:97–106. doi: 10.1016/j.ejmech.2017.11.025. - DOI - PubMed
-
- Mohsin N.-U., Seebacher W., Hochegger P., Faist J., Saf R., Kaiser M., Mäser P., Weis R. Synthesis of new 1-benzyl tetrahydro-4-ylidene piperidinium salts and their antiplasmodial and antitrypanosomal activities. Med. Chem. Res. 2019;28:742–753. doi: 10.1007/s00044-019-02331-7. - DOI
-
- Petrisch M., Seebacher W., Mohsin N.-U., Dolensky J., Hochegger P., Kaiser M., Mäser P., Belaj F., Saf R., Kretschmer N., et al. Preparation of new 1,3-dibenzyl tetrahydropyridinylidene ammonium salts and their antimicrobial and anticellular activities. Eur. J. Med. Chem. 2021;210:112969. doi: 10.1016/j.ejmech.2020.112969. - DOI - PubMed
-
- Sui Z., Flores C., Winters M.P. Cyclopentylpyrazoles as N-Type Calcium Channel Blockers. WO2014/28801. Patent. 2014 October 7;
LinkOut - more resources
Full Text Sources

