Chlorambucil-Bearing Hybrid Molecules in the Development of Potential Anticancer Agents
- PMID: 37836732
- PMCID: PMC10574256
- DOI: 10.3390/molecules28196889
Chlorambucil-Bearing Hybrid Molecules in the Development of Potential Anticancer Agents
Abstract
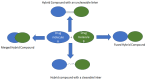
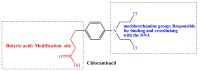
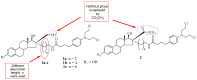
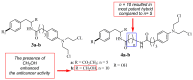
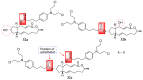
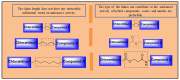
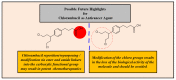
Increasing cases of cancer have been a primary concern in recent decades. Developing new chemotherapeutics is challenging and has been faced with limitations, such as multidrug resistance, poor specificity, selectivity, and toxicity. The aforementioned factors contribute to treatment failure. Hybrid compounds have features that can overcome the limitations mentioned above. Chlorambucil, an anticancer drug that is used to treat prostate and breast cancer, suffers from poor aqueous solubility and specificity, a short half-life, and severe side effects, including anaemia and bone marrow suppression. It compromises the immune system, resulting in treatment failure. Hence, its combination with other pharmacophores has been reported to result in effective anticancer agents with fewer side effects and high therapeutic outcomes. Furthermore, this review gives an update (2010 to date) on the developments of chlorambucil hybrid compounds with anticancer activity, and the structure-activity relationship (SAR), and also highlights future strategies for developing novel anticancer agents.
Keywords: cancer; chlorambucil; drug resistance; hybridization; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
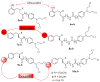
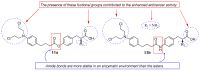
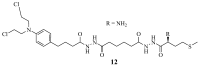
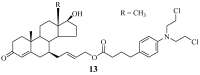
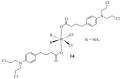
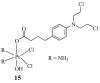
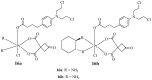
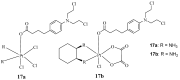

References
-
- Wende M., Sithole S., Chi G.F., Stevens M.Y., Mukanganyama S. The Effects of Combining Cancer Drugs with Compounds Isolated from Combretum zeyheri Sond. and Combretum platypetalum Welw. ex M.A. Lawson (Combretaceae) on the Viability of Jurkat T Cells and HL-60 Cells. Biomed. Res. Int. 2021;2021:6049728. doi: 10.1155/2021/6049728. - DOI - PMC - PubMed
-
- Rashid M., Afzal O., Altamimi S.A.S. Benzimidazole molecule hybrid with oxadiazole ring as antiproliferative agents: In-silico analysis, synthesis and biological evaluation. J. Chil. Chem. Soc. 2021;66:5164–5182. doi: 10.4067/S0717-97072021000205164. - DOI
-
- Chhikara B.S., Parang K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Letter. 2022;10:451
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

