Antioxidant and Anti-Aging Phytoconstituents from Faucaria tuberculosa: In Vitro and In Silico Studies
- PMID: 37836738
- PMCID: PMC10574154
- DOI: 10.3390/molecules28196895
Antioxidant and Anti-Aging Phytoconstituents from Faucaria tuberculosa: In Vitro and In Silico Studies
Abstract
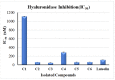
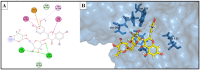
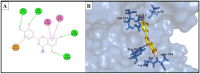
Research targeting natural cosmeceuticals is now increasing due to the safety and/or limited side effects of natural products that are highly valued in cosmetology. Within a research program exploring botanical sources for valuable skincare antioxidant components, the current study investigated the phytochemical content and the biological potential of Faucaria tuberculosa. Phytochemical investigation of F. tuberculosa extract resulted in purification and characterization of six phytoconstituents, including a new one. The structure of the new constituent was elucidated as (-) catechin-(2→1',4→2')-phloroglucinol (4). The structural identity of all isolated compounds were confirmed on the basis of extensive physical and spectral (1D, 2D-NMR and HRESIMS) investigations. The ethanolic extract exhibits a rich content of total phenolics (TPC) and total flavonoids (TFC), estimated as 32 ± 0.034 mg GAE/g and 43 ± 0.004 mg RE/g, respectively. In addition, the antioxidant (ABTS and FRAP), antihyaluronidase and antityrosinase activities of all purified phytoconstituents were evaluated. The results noted (-) catechin-(2→1',4→2') phloroglucinol (4) and phloroglucinol (1) for their remarkable antioxidant activity, while isorhamnetin 3-O-rutinoside (3) and 3,5-dihydroxyphenyl β-D-glucopyranoside (2) achieved the most potent inhibitory activity against tyrosinase (IC50 22.09 ± 0.7 µM and 29.96 ± 0.44 µM, respectively) and hyaluronidase enzymes (IC50 49.30 ± 1.57 µM and 62.58 ± 0.92, respectively) that remarkably exceeds the activity of the standard drugs kojic acid (IC50 = 65.21 ± 0.47 µM) and luteolin, (IC50 = 116.16 ± 1.69 µM), respectively. A molecular docking study of the two active compounds (3 and 2) highlighted their high potential to bind to the active sites of the two enzymes involved in the study.
Keywords: Faucaria tuberculosa; anioxidant; anti-aging; hyaluronidase enzyme; molecular docking simulation; tyrosinase enzyme.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
-
- Bakr R.O. A Comprehensive Review of the Aizoaceae Family: Phytochemical and Biological Studies. Nat. Prod. J. 2021;11:288–304. doi: 10.2174/2210315510666200206154000. - DOI
-
- Glavich T. Beginner’s Guide to Faucaria. Cactus Succul. J. 2016;88:290–292. doi: 10.2985/015.088.0605. - DOI
-
- Schwantes G., Higgins V. Flowering Stones and Mid-Day Flowers: A Book for Plant and Nature Lovers on the Mesembryanthemaceae. E. Benn; London, UK: 1957.
-
- Graf A.B. Pictorial Cyclopedia of Exotic Plants from Tropical and Near-Tropic Regions: Guide to Care of Plants Indoors, Horticultural Color Guide, Plant Geography. Roehr; Cincinnati, OH, USA: 1976.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

